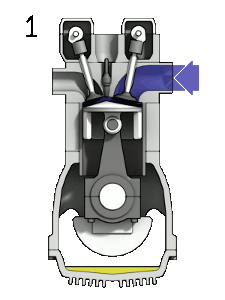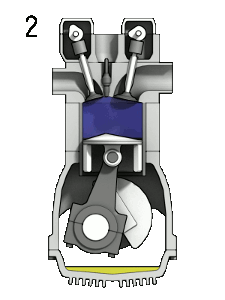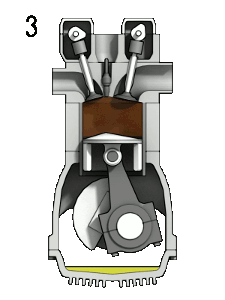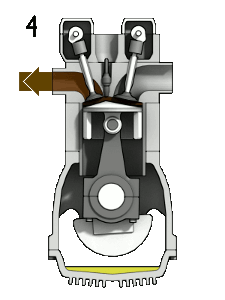
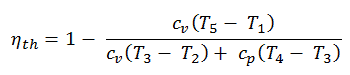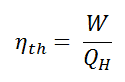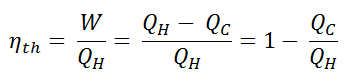Diesel Cycle – Diesel Engine
A dual cycle, or limited pressure cycle, is a thermodynamic cycle that combines the Otto cycle and the Diesel cycle. In the dual cycle, combustion occurs partly at constant volume and partly at constant pressure. It can be used to describe internal combustion engines. The pressure-volume diagrams of actual internal combustion engines are not described well by the Otto and Diesel cycles. An air standard cycle that can be made to approximate the pressure variations more closely is the air-standard dual cycle. A more capable approach would be to model the combustion process in both Otto and Diesel engines as a combination of two heat-transfer processes, one isochoric process, and one isobaric process.
Compared to an Otto cycle, which assumes an instantaneous heat addition (isochoric heat addition), heat is added partly at constant volume and partly at constant pressure in a dual cycle. Therefore the advantage is that more time is available for the fuel to combust completely. On the other hand, the use of a dual cycle is slightly more complex. The thermal efficiency lies between Otto and Diesel cycle.
Thermal Efficiency for Dual Cycle
In general, the thermal efficiency, ηth, of any heat engine is defined as the ratio of the work it does, W, to the heat input at the high temperature, QH.
The thermal efficiency, ηth, represents the fraction of heat, QH, converted to work. Since energy is conserved according to the first law of thermodynamics and energy cannot be converted to work completely, the heat input, QH, must equal the work done, W, plus the heat that must be dissipated as waste heat QC into the environment. Therefore we can rewrite the formula for thermal efficiency as:
Therefore the heat added and rejected are given by:
Qadd-1 = mcv (T3 – T2)
Qadd-2 = mcp (T4 – T3)
Qout = mcv (T5 – T1)
Therefore the thermal efficiency for a dual cycle is: