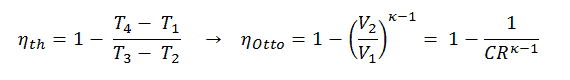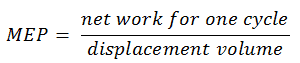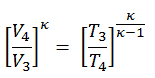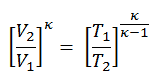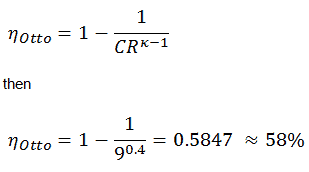Otto Cycle – Otto Engine
In 1876, a German engineer, Nikolaus August Otto, advanced the study of heat engines by building the first working four-stroke engine. A stationary engine using a coal gas-air mixture for fuel. Wilhelm Maybach (1846-1929), one of the most important German engineers, perfected the construction, which was produced in large quantities already at the end of the year 1876. These inventions quickly reshaped the world in which they lived.
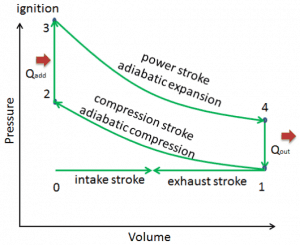
The cycle of the Otto engine is called the Otto cycle. It is one of the most common thermodynamic cycles found in automobile engines and describes the functioning of a typical spark ignition piston engine. In contrast to the Carnot cycle, the Otto cycle does not execute isothermal processes because these must be performed very slowly. In an ideal Otto cycle, the system executing the cycle undergoes a series of four internally reversible processes: two isentropic (reversible adiabatic) processes alternated with two isochoric processes.
Since Carnot’s principle states that no engine can be more efficient than a reversible engine (a Carnot heat engine) operating between the same high temperature and low-temperature reservoirs, the Otto engine must have lower efficiency than the Carnot efficiency. A typical gasoline automotive engine operates at around 25% to 30% of thermal efficiency. About 70-75% is rejected as waste heat without being converted into useful work, i.e., work delivered to wheels.
Otto Cycle – Four Stroke Engine
The Otto cycle is a set of spark-ignition internal combustion engines (two-stroke or four-stroke cycles). Nikolaus August Otto first designed what is known as the four-stroke engine. A stroke refers to the full travel of the piston along with the cylinder in either direction. Therefore each does not correspond to a single thermodynamic process given in chapter Otto Cycle – Processes.
The four-stroke engine comprises:
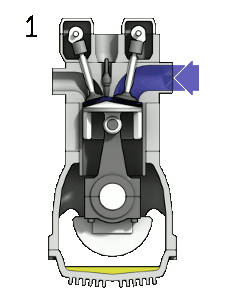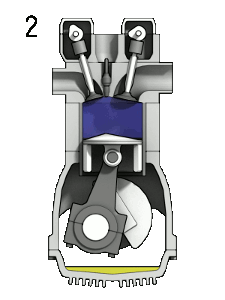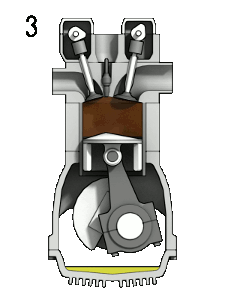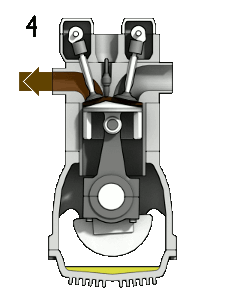
- the intake stroke – The piston moves from the top dead center (TDC) to the bottom dead center (BDC), and the cycle passes 0 → 1. In this stroke, the intake valve is open while the piston pulls an air-fuel mixture into the cylinder by producing vacuum pressure into the cylinder through its downward motion.
- the compression stroke – The piston moves from the bottom dead center (BDC) to the top dead center (TDC), and the cycle passes points 1 → 2 . In this stroke, both the intake and exhaust valves are closed. Therefore the fuel-air mixture is compressed. At the end of this stroke, the fuel-air mixture is ignited by a spark, which causes a further increase in pressure and temperature in the chamber. At the end of this stroke, the crankshaft has completed a full 360-degree revolution.
- the power stroke – The piston moves from the top dead center (TDC) to the bottom dead center (BDC), and the cycle passes points 2 → 3 → 4. In this stroke, both the intake and exhaust valves are closed. At the beginning of the power stroke, a spark ignites the fuel-air mixture in the combustion chamber, which causes very rapid combustion of the fuel. In this stroke, the piston is driven towards the crankshaft, the volume increases, and the pressure falls as work is done by the gas on the piston.
- the exhaust stroke. The piston moves from the bottom dead center (BDC) to the top dead center (TDC), and the cycle passes 4 → 1 → 0. The exhaust valve is open in this stroke while the piston pulls exhaust gases out of the chamber. At the end of this stroke, the crankshaft has completed a second full 360-degree revolution.
Comparison of Actual and Ideal Otto Cycles
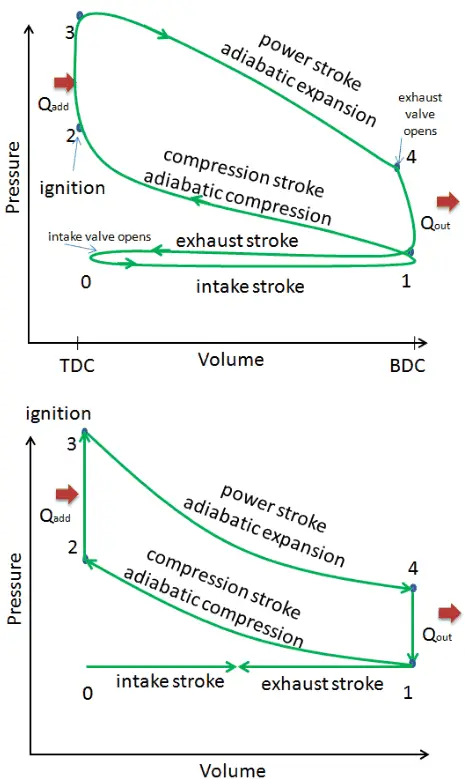 This section shows an ideal Otto cycle in which there are a lot of assumptions that differ from the actual Otto cycle. The main differences between the actual and ideal Otto engine appear in the figure. In reality, the ideal cycle does not occur, and there are many losses associated with each process. For an actual cycle, the shape of the pV diagram is similar to the ideal, but the area (work) enclosed by the pV diagram is always less than the ideal value. The ideal Otto cycle is based on the following assumptions:
This section shows an ideal Otto cycle in which there are a lot of assumptions that differ from the actual Otto cycle. The main differences between the actual and ideal Otto engine appear in the figure. In reality, the ideal cycle does not occur, and there are many losses associated with each process. For an actual cycle, the shape of the pV diagram is similar to the ideal, but the area (work) enclosed by the pV diagram is always less than the ideal value. The ideal Otto cycle is based on the following assumptions:
- Closed cycle. The largest difference between the two diagrams is the simplification of the intake and exhaust strokes in the ideal cycle. In the exhaust stroke, heat Qout is ejected into the environment. In a real engine, the gas leaves the engine and is replaced by a new mixture of air and fuel.
- Instantaneous heat addition (isochoric heat addition). In real engines, the heat addition is not instantaneous. Therefore the peak pressure is not at TDC but just after TDC.
- No heat transfer (adiabatic)
- Compression – The gas (fuel-air mixture) is compressed adiabatically from state 1 to state 2. In real engines, there are always some inefficiencies that reduce the thermal efficiency.
- Expansion. The gas (fuel-air mixture) expands adiabatically from state 3 to state 4.
- Complete combustion of the fuel-air mixture.
- No pumping work. Pumping work is the difference between the work done during the exhaust stroke and the intake stroke. In real cycles, there is a pressure difference between exhaust and inlet pressures.
- No blowdown loss. The early opening of exhaust valves causes blowdown loss. This results in a loss of work output during the expansion stroke.
- No blow-by loss. The blow-by loss is caused by the leakage of compressed gases through piston rings and other crevices.
- No frictional losses.
These simplifying assumptions and losses lead to the fact that the enclosed area (work) of the pV diagram for an actual engine is significantly smaller than the area (work) enclosed by the pV diagram of the ideal cycle. In other words, the ideal engine cycle will overestimate the network and, if the engines run at the same speed, greater power is produced by the actual engine by around 20%.
Compression Ratio – Otto Engine
The compression ratio, CR, is defined as the ratio of the volume at the bottom dead center and the volume at the top dead center. It is a key characteristic of many internal combustion engines. In the following section, it will be shown that the compression ratio determines the thermal efficiency of the used thermodynamic cycle of the combustion engine. It is desired to have a high compression ratio because it allows an engine to reach higher thermal efficiency.
For example, let assume an Otto cycle with compression ratio of CR = 10 : 1. The volume of the chamber is 500 cm³ = 500×10-6 m3 (0.5l) prior to the compression stroke. For this engine all required volumes are known:
- V1 = V4 = Vmax = 500×10-6 m3 (0.5l)
- V2 = V3 = Vmin = Vmax / CR = 55.56 ×10-6 m3
Note that (Vmax – Vmin) x number of cylinders = total engine displacement.
Examples of Compression Ratios – Gasoline vs. Diesel
- The compression ratio in a gasoline-powered engine will usually not be much higher than 10:1 due to potential engine knocking (autoignition) and not lower than 6:1.
- A turbocharged Subaru Impreza WRX has a compression ratio of 8.0:1. In general, turbocharged or supercharged engines already have compressed air at the air intake. Therefore they are usually built with a lower compression ratio.
- A stock Honda S2000 engine (F22C1) has a compression ratio of 11.1:1.
- Some atmospheric sportscar engines can have a ratio of 12.5 : 1 (e.g.,, Ferrari 458 Italia).
- In 2012, Mazda released new petrol engines under the brand name SkyActiv with a 14:1 compression ratio. Residual gas is reduced by using 4-2-1 engine exhaust systems, implementing a piston cavity, and optimizing fuel injection to reduce the risk of engine knocking.
- The Diesel engines have a compression ratio that normally exceeds 14:1, and ratios over 22:1 are also common.
Thermal Efficiency for Otto Cycle
The air-standard Otto cycle thermal efficiency is a function of compression ratio and κ = cp/cv.
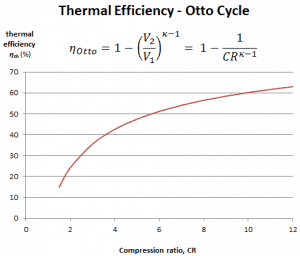
It is a very useful conclusion because it is desirable to achieve a high compression ratio to extract more mechanical energy from a given mass of the air-fuel mixture. A higher compression ratio permits the same combustion temperature to be reached with less fuel while giving a longer expansion cycle. This creates more mechanical power output and lowers the exhaust temperature. Lowering the exhaust temperature causes the lowering of the energy rejected to the atmosphere. This relationship is shown in the figure for κ = 1.4, representing ambient air.
Autoignition – Limit for Compression Ratio
In ordinary gasoline engines, the compression ratio has its limits. The compression ratio in a gasoline-powered engine will usually not be much higher than 10:1 due to potential engine knocking (autoignition) and not lower than 6:1. Higher compression ratios will make gasoline engines subject to engine knocking, caused by the autoignition of an unburned mixture if lower octane-rated fuel is used. The unburned mixture may auto-ignite by detonating from pressure and heat alone rather than igniting the spark plug at exactly the right time. The engine knocking can be reduced by using high-octane fuel, which increases the gasoline’s resistance to autoignition. The higher the octane number, the more compression the fuel can withstand before detonating (igniting). Since the temperature attained by the fuel-air mixture during the compression increases as the compression ratio increases, the likelihood of autoignition increases with the compression ratio. The autoignition can reduce efficiency or damage the engine if knock sensors are not present to modify the ignition timing.
Higher compression ratios can be achieved in diesel engines (also called compression-ignition engines) because they do not compress the fuel but rather compress only air and then inject fuel into the air, which was heated by compression. Compression ratios in the range of 12 to 20 are typical for diesel engines. The greater expansion in diesel engines means they reject less heat in their cooler exhaust. The higher compression ratio (greater expansion) and the higher peak temperature causes that diesel engines reach higher thermal efficiency.
Mean Effective Pressure – MEP
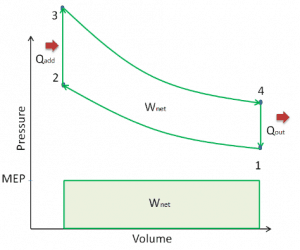
A parameter used by engineers to describe the performance of reciprocating piston engines is known as the mean effective pressure, or MEP. MEP is a useful measure of an engine’s capacity to do work independent of engine displacement. There are several types of MEP. These MEPs are defined by the location measurement and calculation method (e.g.,, BMEP or IMEP).
In general, the mean effective pressure is the theoretical constant pressure. If it acted on the piston during the power stroke, it would produce the same network developed in one complete cycle. The MEP can be defined as:
For example, the net indicated mean effective pressure, known as IMEPn, equals the mean effective pressure calculated from in-cylinder pressure (there must be this measurement) over the complete engine cycle. Note that it is 720° for a four-stroke engine and 360° for a two-stroke engine.
Some examples:
- MEP of an atmospheric gasoline engine can range from 8 to 11 bar in the region of maximum torque.
- MEP of a turbocharged gasoline engine can range from 12 to 17 bar.
- MEP of an atmospheric diesel engine can range from 7 to 9 bar.
- MEP of a turbocharged diesel engine can range from 14 to 18 bar
For example, a four-stroke gasoline engine producing 200 N·m from 2 litres of displacement has a MEP of (4π)(200 N·m)/(0.002 m³) = 1256000 Pa = 12 bar. As can be seen, the MEP is useful characteristics of an engine. For two engines of equal displacement volume, the one with a higher MEP would produce the greater net work and, if the engines run at the same speed, greater power.
Otto Cycle – Problem with Solution
Let assume the Otto cycle, which is one of the most common thermodynamic cycles that can be found in automobile engines. One of the key parameters of such engines is the change in volumes between the top dead center (TDC) to bottom dead center (BDC). The ratio of these volumes (V1 / V2) is known as the compression ratio.
The compression ratio in a gasoline-powered engine will usually not be much higher than 10:1 due to potential engine knocking (autoignition) and not lower than 6:1. For example, some sportscar engines can have a compression ratio up to 12.5 : 1 (e.g.,, Ferrari 458 Italia).
In this example, let assume an Otto cycle with a compression ratio of CR = 9 : 1. The intake air is at 100 kPa = 1 bar, 20 °C, and the chamber volume is 500 cm³ before the compression stroke. The temperature at the end of an adiabatic expansion is T4 = 800 K.
- Specific heat capacity at constant air pressure and room temperature at atmospheric pressure: cp = 1.01 kJ/kgK.
- Specific heat capacity at constant air volume at atmospheric pressure and room temperature: cv = 0.718 kJ/kgK.
- κ = cp/cv = 1.4
Calculate:
- the mass of intake air
- the temperature T3
- the pressure p3
- the amount of heat added by burning of fuel-air mixture
- the thermal efficiency of this cycle
- the MEP
Solution:
1) the mass of intake air
At the beginning of calculations, we must determine the amount of gas in the cylinder before the compression stroke. Using the ideal gas law, we can find the mass:
pV = mRspecificT
where:
- p is the absolute pressure of the gas
- m is the mass of the substance
- T is the absolute temperature
- V is the volume
- Rspecific is the specific gas constant, equal to the universal gas constant divided by the gas or mixture’s molar mass (M). For dry air Rspecific = 287.1 J.kg-1.K-1.
therefore
m = p1V1/RspecificT1 = (100000 × 500×10-6 )/(287.1 × 293) = 5.95×10-4 kg
In this problem all volumes are known:
- V1 = V4 = Vmax = 500×10-6 m3 (0.5l)
- V2 = V3 = Vmin = Vmax / CR = 55.56 ×10-6 m3
Note that (Vmax – Vmin) x number of cylinders = total engine displacement.
2) the temperature T3
Since the process is adiabatic, we can use the following p, V, T relation for adiabatic processes:
thus
T3 = T4 . CRκ – 1 = 800 . 90.4 = 1926 K
3) the pressure p3
Again, we can use the ideal gas law to find the pressure at the beginning of the power stroke as:
p3 = mRspecificT3 / V3 = 5.95×10-4 x 287.1 x 1926 / 55.56 ×10-6 = 5920000 Pa = 59.2 bar
4) the amount of heat added
To calculate the amount of heat added by burning of fuel-air mixture, Qadd, we have to use the first law of thermodynamics for isochoric process, which states the Qadd = ∆U, therefore:
Qadd = mcv (T3 – T2)
The temperature at the end of the compression stroke can be determined using the p, V, T relation for adiabatic processes between points 1 → 2.
T2 = T1 . CRκ – 1 = 293 . 90.4 = 706 K
then
Qadd = mcv (T3 – T2) = 5.95×10-4 x 718 x 1220 = 521.2 J
5) the thermal efficiency
Thermal efficiency for an Otto cycle:
As was derived in the previous section, the thermal efficiency of an Otto cycle is a function of compression ratio and κ:
6) the mean effective pressure
The MEP was defined as:
In this equation, the displacement volume is equal to Vmax – Vmin. The network for one cycle can be calculated using the heat added and the thermal efficiency:
Wnet = Qadd . ηOtto = 521.2 x 0.5847 = 304.7 J
MEP = 304.7 / (500×10-6 – 55.56 ×10-6) = 685.6 kPa = 6.856 bar