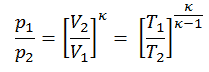Adiabatic Process
An adiabatic process is a thermodynamic process in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated. In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important since we can use the adiabatic approximation only in very rapid processes. There is not enough time for the transfer of energy as heat to take place to or from the system in these rapid processes.
In real devices (such as turbines, pumps, and compressors) heat losses and losses in the combustion process occur, but these losses are usually low in comparison to overall energy flow and we can approximate some thermodynamic processes by the adiabatic process.
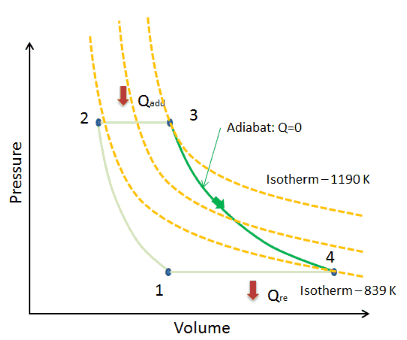
Adiabatic Curve – Adiabat
See also: What is an Ideal Gas.
In an ideal gas, molecules have no volume and do not interact. According to the ideal gas law, pressure varies linearly with temperature and quantity and inversely with volume.
pV = nRT
where:
- p is the absolute pressure of the gas
- n is the amount of substance
- T is the absolute temperature
- V is the volume
- R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
In this equation, the symbol R is a constant called the universal gas constant that has the same value for all gases—namely, R = 8.31 J/mol K.
The adiabatic process can be expressed with the ideal gas law as:
pVκ = constant
or
p1V1κ = p2V2κ
in which κ = cp/cv is the ratio of the specific heats (or heat capacities) for the gas. One for constant pressure (cp) and one for constant volume (cv). Note that this ratio κ = cp/cv is a factor in determining the speed of sound in gas and other adiabatic processes.
Other p, V, T Relation
On a p-V diagram, the process occurs along a line (called an adiabat) that has the equation p = constant / Vκ. For an ideal gas and a polytropic process, the case n = κ corresponds to an adiabatic process.