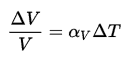Pure heavy water (D2O) has a density about 11% greater than water but is otherwise physically and chemically similar.
The fact causes this difference, and the deuterium nucleus is twice as heavy as the hydrogen nucleus. Since about 89% of the molecular weight of water comes from the single oxygen atom rather than the two hydrogen atoms, the weight of a heavy water molecule is not substantially different from that of a normal water molecule. The molar mass of water is M(H2O) = 18.02, and the molar mass of heavy water is M(D2O) = 20.03 (each deuterium nucleus contains one neutron in contrast to the hydrogen nucleus). Therefore heavy water (D2O) has a density about 11% greater (20.03/18.03 = 1.112).
Pure heavy water (D2O) has its highest density of 1106 kg/m3 at a temperature of 11.6oC (52.9oF). Also, heavy water differs from most liquids in that it becomes less dense as it freezes. It has a maximum density of 11.6oC (1106 kg/m3), whereas its solid form ice density is 1017 kg/m3. It must be noted, the change in density is not linear with temperature because the volumetric thermal expansion coefficient for water is not constant over the temperature range.