Source: wikipedia.org CC BY-SA
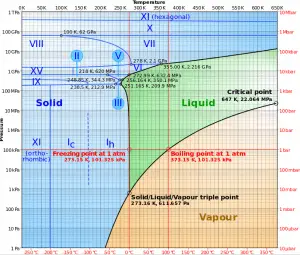
At pressure that is higher than the critical pressure, water is in a special state, which is known as the supercritical fluid state. A supercritical fluid is a fluid that is at pressures higher than its thermodynamic critical values. At the critical and supercritical pressures, a fluid is considered a single-phase substance, although all thermophysical properties undergo significant changes within the critical and pseudocritical regions.
For water, the critical parameters are the following:
- Pcr = 22.09 MPa
- Tcr = 374.14 °C (or 647.3 K)
- vcr = 0.003155 m3/kg
- uf = ug = 2014 kJ/kg
- hf = hg = 2084 kJ/kg
- sf = sg =4.406 kJ/kg K
See also: Critical Point of Water
See also: Supercritical Fluid