What is Pressure
 Pressure is a measure of the force exerted per unit area on the boundaries of a substance. The standard unit for pressure in the SI system is the Newton per square meter or pascal (Pa). Mathematically:
Pressure is a measure of the force exerted per unit area on the boundaries of a substance. The standard unit for pressure in the SI system is the Newton per square meter or pascal (Pa). Mathematically:
p = F/A
where
- p is the pressure
- F is the normal force
- A is the area of the boundary
Pascal is defined as a force of 1N that is exerted on a unit area.
- 1 Pascal = 1 N/m2
- 1 MPa 106 N/m2
- 1 bar 105 N/m2
- 1 kPa 103 N/m2
In general, pressure or the force exerted per unit area on the boundaries of a substance is caused by the collisions of the molecules of the substance with the boundaries of the system. As molecules hit the walls, they exert forces that try to push the walls outward. The forces resulting from all of these collisions cause the pressure exerted by a system on its surroundings. Pressure as an intensive variable is constant in a closed system. It is only relevant in liquid or gaseous systems.
SI Unit of Pressure – Pascal – kg/m.s2
Pascal – Unit of Pressure
As was discussed, the SI unit of pressure and stress is the pascal.
- 1 pascal 1 N/m2 = 1 kg / (m.s2)
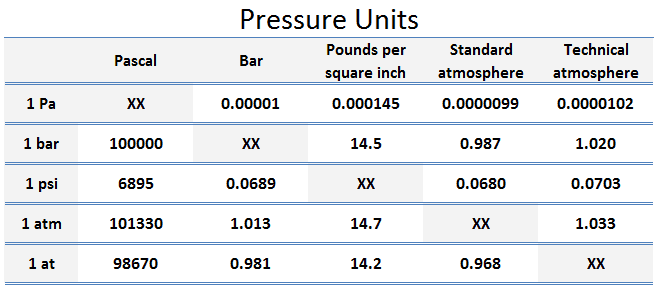
Pascal is defined as one Newton per square meter. However, it is a fairly small unit for most engineering problems, so it is convenient to work with multiples of the pascal: the kPa, the bar, and the MPa.
- 1 MPa 106 N/m2
- 1 bar 105 N/m2
- 1 kPa 103 N/m2
The unit of measurement called standard atmosphere (atm) is defined as:
- 1 atm = 101.33 kPa
The standard atmosphere approximates the average pressure at sea level at the latitude of 45° N. Note that there is a difference between the standard atmosphere (atm) and the technical atmosphere (at).
A technical atmosphere is a non-SI unit of pressure equal to one kilogram-force per square centimeter.
- 1 at = 98.67 kPa