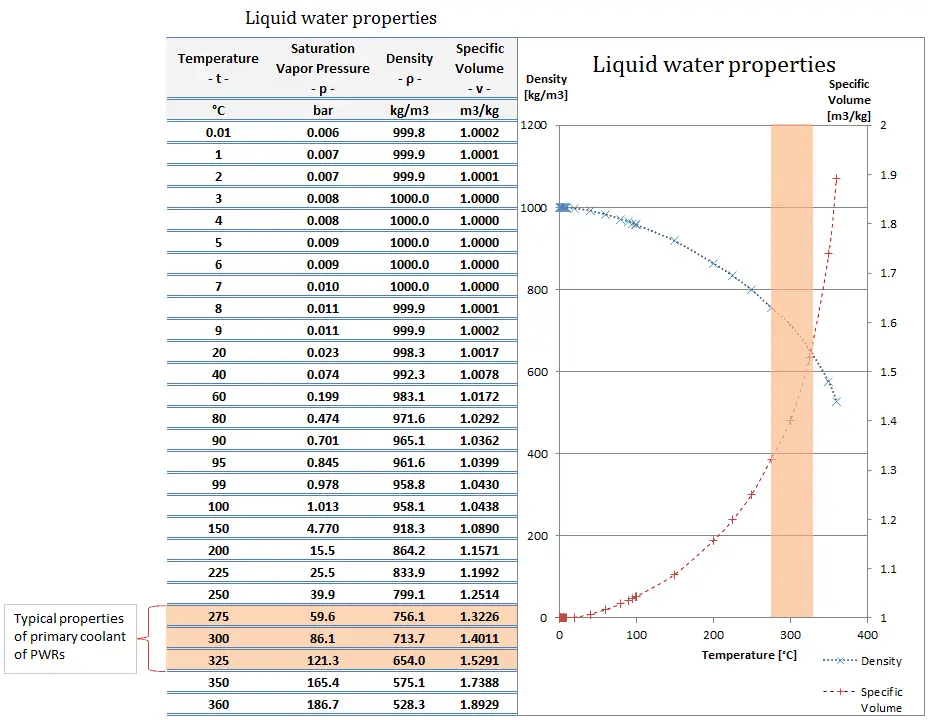
Specific volume is an intensive variable, whereas volume is an extensive variable. The standard unit for specific volume in the SI system is cubic meters per kilogram (m3/kg). The standard unit in the English system is cubic feet per pound-mass (ft3/lbm).
The density (ρ) of a substance is the reciprocal of its specific volume (ν).
ρ = m/V = 1/ρ
Density is defined as the mass per unit volume. It is also an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
Densest Materials on the Earth – Materials with Lowest Specific Volume
Since nucleons (protons and neutrons) make up most of the mass of ordinary atoms, the density of normal matter tends to be limited by how closely we can pack these nucleons and depends on the internal atomic structure of a substance. The densest material found on earth is the metal osmium. Still, its density pales by comparison to the densities of exotic astronomical objects such as white dwarf stars and neutron stars.
List of densest materials:
- Osmium – 22.6 x 103 kg/m3
- Iridium – 22.4 x 103 kg/m3
- Platinum – 21.5 x 103 kg/m3
- Rhenium – 21.0 x 103 kg/m3
- Plutonium – 19.8 x 103 kg/m3
- Gold – 19.3 x 103 kg/m3
- Tungsten – 19.3 x 103 kg/m3
- Uranium – 18.8 x 103 kg/m3
- Tantalum – 16.6 x 103 kg/m3
- Mercury – 13.6 x 103 kg/m3
- Rhodium – 12.4 x 103 kg/m3
- Thorium – 11.7 x 103 kg/m3
- Lead – 11.3 x 103 kg/m3
- Silver – 10.5 x 103 kg/m3
It must be noted, plutonium is a manufactured isotope and is created from uranium in nuclear reactors. But scientists have found trace amounts of naturally-occurring plutonium.
If we include manufactured elements, the densest so far is Hassium. Hassium is a chemical element with the symbol Hs and atomic number 108. It is a synthetic element (first synthesized at Hasse in Germany) and radioactive. The most stable known isotope, 269Hs, has a half-life of approximately 9.7 seconds. It has an estimated density of 40.7 x 103 kg/m3. The density of Hassium results from its high atomic weight and the significant decrease in ionic radii of the elements in the lanthanide series, known as lanthanide and actinide contraction.
The density of Hassium is followed by Meitnerium (element 109, named after the physicist Lise Meitner), which has an estimated density of 37.4 x 103 kg/m3.