Temperature Scales
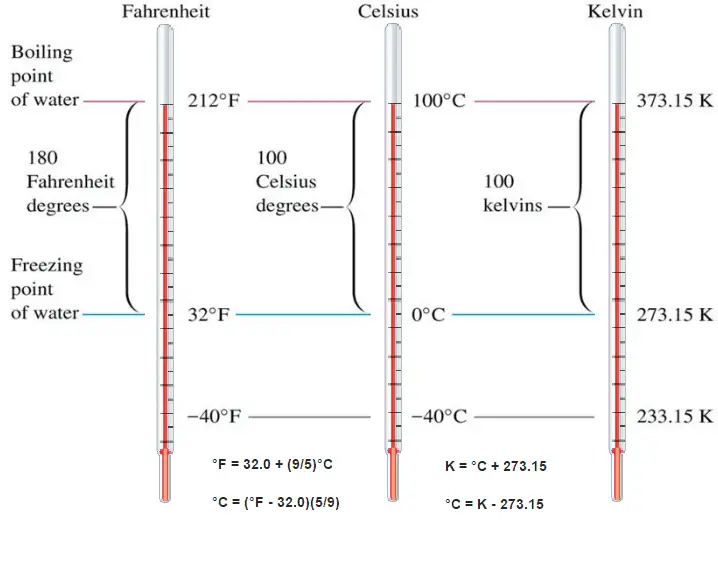 When using a thermometer, we need to mark a scale on the tube wall with numbers on it. We have to define a temperature scale. A temperature scale is a way to measure temperature relative to a starting point (0 or zero) and a unit of measurement.
When using a thermometer, we need to mark a scale on the tube wall with numbers on it. We have to define a temperature scale. A temperature scale is a way to measure temperature relative to a starting point (0 or zero) and a unit of measurement.
These numbers are arbitrary, and historically many different schemes have been used. For example, this was done by defining some physical occurrences at given temperatures—such as the freezing and boiling points of water — and defining them as 0 and 100, respectively.
There are several scales and units exist for measuring temperature. The most common are:
Absolute Zero
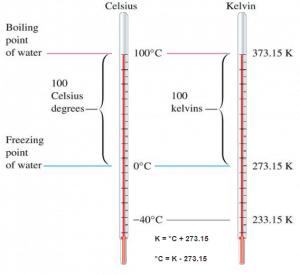
The absolute temperature scale that corresponds to the Celsius scale is called the Kelvin (K) scale, and the absolute scale that corresponds to the Fahrenheit scale is called the Rankine (R) scale. The zero points on both absolute scales represent the same physical state. The relationships between the absolute and relative temperature scales are shown in the following equations.
Kelvin – Celsius
K = °C + 273.15
°C = K – 273.15
Rankine – Fahrenheit
R = °F + 460
°F = R – 460
Absolute Zero
Such a scale has as its zero point. The coldest theoretical temperature is called absolute zero, at which the thermal motion of atoms and molecules reaches its minimum. This is a state at which the enthalpy and entropy of a cooled ideal gas reach its minimum value, taken as 0. Classically, this would be a state of motionlessness, but quantum uncertainty dictates that the particles still possess finite zero-point energy. Absolute zero is denoted as 0 K on the Kelvin scale, −273.15 °C on the Celsius scale, and −459.67 °F on the Fahrenheit scale.
Absolute Zero and Third Law of Thermodynamics
Third law of thermodynamics states:
The entropy of a system approaches a constant value as the temperature approaches absolute zero.
Based on empirical evidence, this law states that the entropy of a pure crystalline substance is zero at the absolute zero of temperature, 0 K and that it is impossible using any process, no matter how idealized, to reduce the temperature of a system to absolute zero in a finite number of steps. This allows us to define a zero point for the thermal energy of a body.
Lowest manufactured temperature
According to Guiness World Records the lowest manmade temperature achieved to date is 450 picokelvin above absolute zero (only half-a-billionth of a degree above absolute zero). It was achieved by a team of scientists at the Massachusetts Institute of Technology, Cambridge, Massachusetts, USA.