If two systems are both in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
We can discover an important property of thermal equilibrium by considering three systems. A, B, and C are initially not in thermal equilibrium. We separate systems A and B with an adiabatic wall (ideal insulating material), but we let system C interact with systems A and B. We wait until thermal equilibrium is reached; then, A and B are in thermal equilibrium with C. But are they in thermal equilibrium with each other?
According to many experiments, there will be no net energy flow between A and B. This is experimental evidence of the following statement:
If two systems are both in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
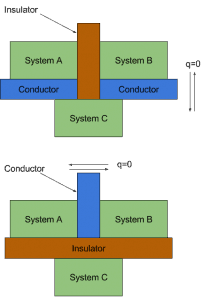
This statement is known as the zeroth law of thermodynamics. It has this unusual name because it was not until scientists realized that this obvious postulate needed to be stated first after the great first and second laws of thermodynamics were worked out.
 This law provides a definition and method of defining temperatures, perhaps the most important intensive property of a system when dealing with thermal energy conversion problems. Temperature is a system property that determines whether the system will be in thermal equilibrium with other systems. When two systems are in thermal equilibrium, their temperatures are, by definition, equal, and no net thermal energy will be exchanged between them. Thus the importance of the zeroth law is that it allows a useful definition of temperature.
This law provides a definition and method of defining temperatures, perhaps the most important intensive property of a system when dealing with thermal energy conversion problems. Temperature is a system property that determines whether the system will be in thermal equilibrium with other systems. When two systems are in thermal equilibrium, their temperatures are, by definition, equal, and no net thermal energy will be exchanged between them. Thus the importance of the zeroth law is that it allows a useful definition of temperature.