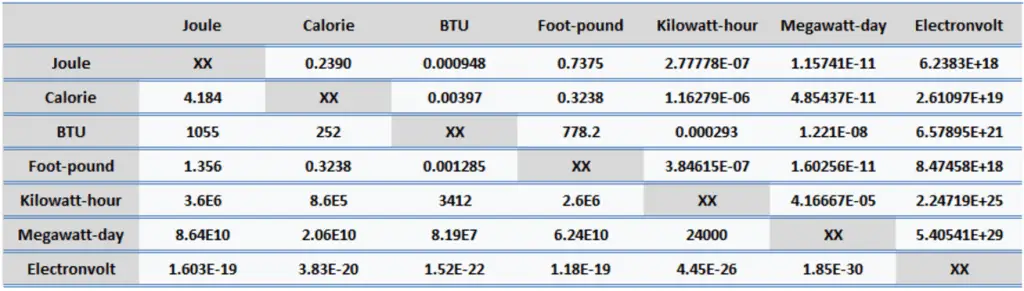
Energy is generally defined as the potential to do work or produce heat. This definition causes the SI unit for energy to be the same as the unit of work – the joule (J). Joule is a derived unit of energy, and it is named in honor of James Prescott Joule and his experiments on the mechanical equivalent of heat. In more fundamental terms, 1 joule is equal to:
1 J = 1 kg.m2/s2
Since energy is a fundamental physical quantity and is used in various physical and engineering branches, there are many energy units in physics and engineering.
Joule – Energy Units
Joule (unit: J). Joule is a derived unit of energy. It is equal to the energy transferred to an object when a force of one newton acts on that object in the direction of its motion through a distance of one meter.
-
- 1 joule = 0.239 Calories
- 1 joule = 9.48 x 10-4 BTU
- 1 joule = 2.778 x 10-7 kWh
Examples of Energy of 1 Joule
One joule in everyday life and science corresponds to approximately:
- The kinetic energy of an object with mass 1 kg moving at √2 ≈ 1.4 m/s.
- The kinetic energy of a 50 kg object (e.g.,, human) moving very slowly – approximately 0.72 km/h.
- The energy required to lift a medium-size apple (100 g) 1 meter vertically from the surface of the Earth.
- The heat required to raise the temperature of 1 g of water by 0.24 °C.
- The heat required to evaporate of 0.00044 g of liquid water at 100°C.
- The amount of electricity required to light a 1 watt LED for 1 s.
- Is released by approximately 3.1⋅1010 fissions in a nuclear reactor.