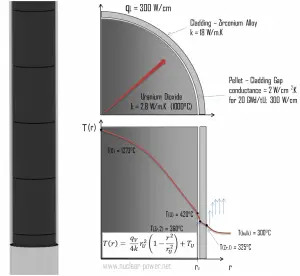
 Fuel cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e., fuel pellets). It is a corrosion-resistant material with a low absorption cross section for thermal neutrons (~ 0.18 × 10–24 cm2), usually zirconium alloy. Cladding prevents radioactive fission products from escaping the fuel matrix into the reactor coolant and contaminating it. Cladding constitutes one of the barriers in the ‘defense-in-depth ‘approach, and therefore its coolability is one of the key safety aspects.
Fuel cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e., fuel pellets). It is a corrosion-resistant material with a low absorption cross section for thermal neutrons (~ 0.18 × 10–24 cm2), usually zirconium alloy. Cladding prevents radioactive fission products from escaping the fuel matrix into the reactor coolant and contaminating it. Cladding constitutes one of the barriers in the ‘defense-in-depth ‘approach, and therefore its coolability is one of the key safety aspects.
Oxidation of Zirconium Alloys
The oxidation of zirconium alloys is one of the most studied processes in the entire nuclear industry. While many of these reports are written to address the reaction of fuel and steam with zirconium alloys in the case of a nuclear accident, there are still a substantial number of reports dealing with the oxidation of zirconium alloys at moderate temperatures of about 800 K and below.
The aggressive agent in this respect is primary circuit water, at a temperature of some 300° C. This oxidizes zirconium according to the reaction:
Zr + 2H2O→ZrO2 + 2H2
resulting in the formation of solid oxide on the metal’s surface. In summary, in the absence of neutron irradiation, the overall oxidation of zirconium and its alloys is fairly well understood. The oxidation rate depends upon the pressure of the oxygen or water vapor and scales approximately with pressure to the 1/6th power. The dependence of oxidation rate R on temperature and pressure can be expressed as:
R = 13.9·P1/6·exp(−1.47/kBT)
The oxidation rate R is here expressed in gram/(cm2·second); P is the pressure in the atmosphere, that is, the factor P1/6 = 1 at ambient pressure; the activation energy is 1.47 eV; kB is the Boltzmann constant (8.617×10−5 eV/K), and T is the absolute temperature in kelvins.
The enhancement in the oxidation rate in the presence of fast neutrons depends upon neutron intensity, temperature, water chemistry, dissolved oxygen in the water, and oxide layer thickness. Part of the hydrogen thus generated is incorporated into the metallic matrix, migrating under the effect of the thermal gradient to accumulate in the less hot regions, forming hydrides that are liable to cause brittleness in the cladding as the fuel cools down. The two processes, oxidation, and hydridation, are thus intimately bound together.
To date, the most commonly used alloy in PWRs has been Zircaloy 4. However, currently, this is being replaced by new zirconium–niobium-based alloys, exhibiting better corrosion resistance. The maximum temperature, at which zirconium alloys can be used in water-cooled reactors depends on their corrosion resistance. Alloys of type Zircalloy, in which tin is the basic alloying element that improves their mechanical properties, have a wide distribution worldwide. However, in this case, the corrosion resistance in water and steam is decreased, resulting in the need for additional alloying. The improvement brought about by the additive niobium probably involves a different mechanism. The high corrosion resistance of niobium alloyed metals in water and steam at temperatures of 400–550°C is caused by their ability to passivation with the formation of protective films.
High-Temperature Steam Oxidation of Zirconium Alloys
As was written in the previous section, the oxidation of zirconium alloys is one of the most studied processes in the nuclear industry. While many of these reports are written to address the reaction of fuel and steam with zirconium alloys in the case of a nuclear accident, there are still a substantial number of reports dealing with the oxidation of zirconium alloys at moderate temperatures of about 800 K and below. The aggressive agent in this respect is primary circuit water, at a temperature of some 300° C. This oxidizes zirconium according to the reaction:
Zr + 2H2O→ZrO2 + 2H2
resulting in the formation of solid oxide on the metal’s surface. The reaction is always exothermic, and the heat of the reaction is similar for all types of zirconium-based alloys. The rate of reaction increases smoothly, although strongly, with temperature.
At high temperatures, the exothermic reaction of Zr-base alloys with steam is much more intensive and hazardous for the safety of nuclear power plants during accidents like a loss-of-coolant accident (LOCA). The main problem of high-temperature oxidation is that zirconium cladding rapidly reacts with water steam at high temperatures. The oxidation kinetics of relevant zirconium alloys appears to be parabolic in the temperature range of 1000-1500°C for many Zr-based alloys. Above 1577°C, the oxide layer transforms from tetragonal to cubic, and the oxidation rate even increases. Moreover, the oxidation of zirconium by water is accompanied by a release of hydrogen gas. This oxidation is accelerated at high temperatures, e.g., inside a reactor core, if the fuel assemblies are no longer completely covered by liquid water and insufficiently cooled. Metallic zirconium is then oxidized by water/steam to form hydrogen gas according to the following redox reaction:
Zr + 2H2O→ZrO2 + 2H2 (Q = 190 kJ/mol; Baker and Just)
Zirconium cladding in the presence of D2O deuterium oxide is frequently used as the moderator and coolant in the next-gen pressurized heavy water reactors that CANDU-designed nuclear reactors use would express the same oxidation on exposure to deuterium oxide steam as follows:
Zr + 2D2O→ZrO2 + 2D2
Although only occurring at high temperatures, this exothermic reaction is similar to alkali metals (such as sodium or potassium) with water.
Above approximately 800°C, there is a phase transformation from the hexagonal (HCP) α-phase to the cubic β-phase (BCC). Oxygen, which diffuses into the remaining metal, stabilizes the α-phase. With cooling, the β phase will transform back to the α phase in which the oxygen concentration is significantly different from that of the oxygen-stabilized α and “prior β.” The problem is that these phase transitions make this material very brittle. Therefore, there are also three current LOCA licensing criteria (according to 10 CFR 50.46 – ECCS Acceptance Criteria for LWR) for preventing fuel failure (it can fail upon rewet during the introduction of emergency core cooling in a reactor accident or fail by overstraining under oxide cracks) due to oxygen and hydrogen embrittlement and these criteria limit:
- Maximum Cladding Oxidation. The calculated total oxidation of the cladding shall nowhere exceed 0.17 times the total cladding thickness before oxidation. In other words, this criterion limits maximum Equivalent Cladding Reacted (ECR) to 17% during high-temperature steam oxidation to ensure adequate ductility during the Emergency Core Cooling System (ECCS) quench and possible post-LOCA seismic events. After the 1973 ECCS hearing, the Commission’s opinion was that retention of ductility in the Zircaloy cladding was the best guarantee of its remaining intact during a loss-of-coolant accident. The original 17% maximum cladding oxidation limit was derived from ring compression tests performed on oxidized rings of Zircaloy cladding that were intended to show under what temperature and oxidation conditions residual ductility would be present after a LOCA. During a loss-of-coolant accident (LOCA), fuel rods are expected to balloon, rupture, and then oxidize as the temperature rises toward the peak cladding temperature. Oxidation inside the cladding is also expected in the ruptured region, and maximum cladding oxidation is always found in that location. Regulation §50.46 requires the calculation of double-sided oxidation in the ruptured region, which is this amount of oxidation, and it is compared with the 17% limit to demonstrate ductility.
- Peak Cladding Temperature. The calculated maximum fuel element cladding temperature shall not exceed 2200°F (1204°C). This criterion ensures the validity of the ECR criterion.
- Parabolic oxidation kinetics. The oxidation kinetics of relevant zirconium alloys appears to be parabolic in the temperature range of 1000-1500°C for many Zr-based alloys. During the period when high cladding temperatures occur, the heat sources are decay heat and oxidation heat. As temperature increases, the oxidation heat becomes dominant such that the entire heat source increases rapidly with temperature. At approximately 2200°F, the oxidation heat (generated from typical zirconium alloys) equals the decay heat generated after 8 hours from reactor shutdown.
- Phase transition and additional embrittlement. As was written, Above approximately 800°C, there is a phase transformation from the hexagonal (HCP) α-phase to the cubic β-phase (BCC). Oxygen, which diffuses into the remaining metal, stabilizes the α-phase. The problem is that above 2200°F, these phase transitions and oxygen concentration make this material very brittle. Above 1577°C, the oxide layer transforms from tetragonal to cubic, and the oxidation rate even increases, but this is well above the range of interest for a limit. Below 1000°C, the weight gain deviates from parabolic behavior as the alloys transform from the β-phase to the mixed β+α-phase to the α-phase. Phase transformation temperatures depend on alloy type, oxygen, and hydrogen concentrations. Therefore, peak cladding temperature was discussed extensively in the 1973 ECCS hearing, and the 2200°F value arose in connection with the 17% maximum cladding oxidation value. The 2200°F value was chosen because the relation between maximum cladding oxidation and ductility broke down for temperatures between 2200 and 2400°F (no data in between). It would, of course, have been possible to choose a higher peak cladding temperature and a correspondingly lower maximum cladding oxidation value.
- Maximum hydrogen generation. The calculated total amount of hydrogen generated from the chemical reaction of the cladding with water or steam shall not exceed 0.01 times the hypothetical amount that would be generated if all of the metal in the cladding cylinders surrounded the fuel, excluding the cladding surrounding the plenum volume, were to react.
Note that loss-of-coolant accidents (LOCA’s) are hypothetical accidents that would result from the loss of reactor coolant, at a rate over the capability of the reactor coolant makeup system, from breaks in pipes in the reactor coolant pressure boundary up to and including a break equivalent in size to the double-ended rupture of the largest pipe in the reactor coolant system.
Special Reference: Baker, L., Just, L.C., Studies of Metal Water Reactions at High Temperatures, III. Experimental and Theoretical Studies of the Zirconium-Water Reaction, ANL-6548, page 7, May 1962
Special Reference: M.C. Billone, H.M. Chung, and Y. Yan. STEAM OXIDATION KINETICS OF ZIRCONIUM ALLOYS. Argonne National Laboratory, June 4, 2002.
Special Reference: F.C. Iglesias, B.J. Lewis, C. Desgranges, C. Toffolon. Clad-coolant chemical interaction. NEA/NSC/R-2015-5. NEA, OECD, 2015.
Baker-Just Correlation
In 1973, the Baker-Just correlation was selected as the best correlation available and was included as a requirement in Appendix K of 10 CFR 50.46 for calculating the heating rate due to oxidation, hydrogen generation, and the Effective Cladding Reacted (ECR) because it was available in 1973. The Baker-Just equation and more recent correlations can be represented as an Arrhenius relation (e-Q/RT) with an activation energy Q and an exponential dependence on temperature T (R is the universal gas constant). Thus, the oxidation rate and, hence, the heat generation rate increase rapidly as temperature increases. The integrated form of the Baker-Just correlation (BJ) for weight/surface-area (w) of zirconium consumed during steam oxidation is
w2 = 33.3×106 t exp(-45500/RT), (mg/cm2)2
where t is time in seconds, T is the temperature in K, and R = 1.987. This equation applies to isothermal oxidation in steam for fresh cladding with no protective, pre-transient oxide layer. However, this correlation has the least significant database and justification of all those reviewed. Oxidation kinetics studies on various zirconium alloys conducted since 1962 – particularly in the 1970s – have demonstrated that the Baker-Just correlation over-predicts weight gain and zirconium consumed by as much as 30% at the peak cladding temperature (1204°C) allowed by 10CFR50.46. USNRC Regulatory Guide 1.157 (May 1989) allows the use of a best-estimate correlation (e.g., Cathcart-Pawel) for temperatures greater than 1078°C.
Special Reference: Baker, L., Just, L.C., Studies of Metal Water Reactions at High Temperatures, III. Experimental and Theoretical Studies of the Zirconium-Water Reaction, ANL-6548, page 7, May 1962
Special Reference: M.C. Billone, H.M. Chung, and Y. Yan. STEAM OXIDATION KINETICS OF ZIRCONIUM ALLOYS. Argonne National Laboratory, June 4, 2002.
Special Reference: F.C. Iglesias, B.J. Lewis, C. Desgranges, C. Toffolon. Clad-coolant chemical interaction. NEA/NSC/R-2015-5. NEA, OECD, 2015.