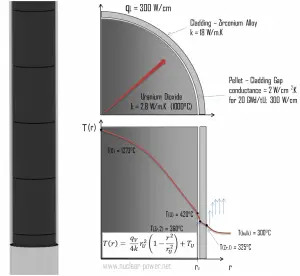
 Fuel cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e., fuel pellets). It is a corrosion-resistant material with a low absorption cross section for thermal neutrons (~ 0.18 × 10–24 cm2), usually zirconium alloy. Cladding prevents radioactive fission products from escaping the fuel matrix into the reactor coolant and contaminating it. Cladding constitutes one of the barriers in the ‘defense-in-depth ‘ approach, and therefore its coolability is one of the key safety aspects.
Fuel cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e., fuel pellets). It is a corrosion-resistant material with a low absorption cross section for thermal neutrons (~ 0.18 × 10–24 cm2), usually zirconium alloy. Cladding prevents radioactive fission products from escaping the fuel matrix into the reactor coolant and contaminating it. Cladding constitutes one of the barriers in the ‘defense-in-depth ‘ approach, and therefore its coolability is one of the key safety aspects.
Zirconium Alloy
A typical composition of nuclear-grade zirconium alloys is more than 95 weight percent zirconium and less than 2% of tin, niobium, iron, chromium, nickel, and other metals added to improve mechanical properties and corrosion resistance. To date, the most commonly used alloy in PWRs has been Zircaloy 4. However, currently, this is being replaced by new zirconium–niobium-based alloys, exhibiting better corrosion resistance. The maximum temperature at which zirconium alloys can be used in water-cooled reactors depends on their corrosion resistance. Alloys of type Zircalloy, in which tin is the basic alloying element that improves their mechanical properties, have a wide distribution worldwide. However, in this case, the decrease of corrosion resistance in water and steam is taken place, resulting in the need for additional alloying. The improvement brought about by the additive niobium probably involves a different mechanism. The high corrosion resistance of niobium alloyed metals in water and steam at temperatures of 400–550°C is caused by their ability to passivation with the formation of protective films.
Oxidation of Zirconium Alloys
The oxidation of zirconium alloys is one of the most studied processes in the entire nuclear industry. While many of these reports are written to address the reaction of fuel and steam with zirconium alloys in the case of a nuclear accident, there are still a substantial number of reports dealing with the oxidation of zirconium alloys at moderate temperatures of about 800 K and below.
The aggressive agent in this respect is primary circuit water, at a temperature of some 300° C. This oxidizes zirconium according to the reaction:
Zr + 2H2O→ZrO2 + 2H2
resulting in the formation of solid oxide on the metal’s surface. In summary, in the absence of neutron irradiation, the overall oxidation of zirconium and its alloys is fairly well understood. The oxidation rate depends upon the pressure of the oxygen or water vapor and scales approximately with pressure to the 1/6th power. The dependence of oxidation rate R on temperature and pressure can be expressed as:
R = 13.9·P1/6·exp(−1.47/kBT)
The oxidation rate R is here expressed in gram/(cm2·second); P is the pressure in the atmosphere, that is, the factor P1/6 = 1 at ambient pressure; the activation energy is 1.47 eV; kB is the Boltzmann constant (8.617×10−5 eV/K), and T is the absolute temperature in kelvins.
The enhancement in the oxidation rate in the presence of fast neutrons depends upon neutron intensity, temperature, water chemistry, dissolved oxygen in the water, and oxide layer thickness. Part of the hydrogen thus generated is incorporated into the metallic matrix, migrating under the effect of the thermal gradient to accumulate in the less hot regions, forming hydrides that are liable to cause brittleness in the cladding as the fuel cools down. The two processes, oxidation, and hydridation, are thus intimately bound together.
To date, the most commonly used alloy in PWRs has been Zircaloy 4. However, currently, this is being replaced by new zirconium–niobium-based alloys, exhibiting better corrosion resistance. The maximum temperature at which zirconium alloys can be used in water-cooled reactors depends on their corrosion resistance. Alloys of type Zircalloy, in which tin is the basic alloying element that improves their mechanical properties, have a wide distribution worldwide. However, in this case, the decrease of corrosion resistance in water and steam is taken place, resulting in the need for additional alloying. The improvement brought about by the additive niobium probably involves a different mechanism. The high corrosion resistance of niobium alloyed metals in water and steam at temperatures of 400–550°C is caused by their ability to passivation with the formation of protective films.