Since natural uranium contains only 0.71% of fissile isotope 235U and most current power reactors require enriched uranium, this natural uranium must be enriched. Only uranium 235 is a fissile material (i.e., it can undergo nuclear fission only after capturing a thermal neutron). Still, as it only accounts for 0.7% of the makeup of natural uranium, it is not present in sufficient proportions to be used in LWR-type nuclear power plants. The level of enrichment required depends on the specific reactor design (e.g., PWRs and BWRs require 3% – 5% of 235U) and specific requirements of the nuclear power plant operator (e.g., cycle length). Without required enrichment, these reactors cannot initiate and sustain a nuclear chain reaction for as long as 12 months (or more).
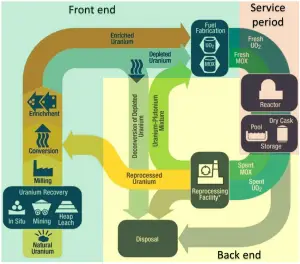
Enrichment is accomplished using any of several methods of isotope separation. Most commercial uranium enrichment processes (gaseous diffusion and the gas centrifuge method) require the uranium to be in a gaseous form. Therefore, the uranium oxide concentrate must first be converted to uranium hexafluoride, a gas at relatively low temperatures. Therefore the input material in this process is uranium hexafluoride, also known as “hex.” In the enrichment process, gaseous uranium hexafluoride is separated into two streams, one being enriched to the required level and known as low-enriched uranium; the other stream is progressively depleted in uranium-235 and is called ‘tails,’ or simply depleted uranium. The product of this stage of the nuclear fuel cycle is enriched uranium hexafluoride, which is reconverted to produce enriched uranium oxide. From a non-proliferation standpoint, uranium enrichment is a sensitive technology needing to be subject to tight international control.
Before enrichment, natural uranium consists primarily of isotope 238U (99.28%). Therefore the atomic mass of the uranium element is close to the atomic mass of the 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). The abundance of isotopes in nature is caused by differences in the half-lives. All three naturally-occurring isotopes of uranium (238U, 235U, and 234U) are unstable. On the other hand, these isotopes (except 234U) belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.5×109years for 238U).
About 10 kg of natural uranium is required with a byproduct of about 9 kg of depleted uranium to produce 1 kg of enriched uranium with 5% of 235U. Therefore annual natural uranium consumption of a 3000 MWth reactor is about 250 tonnes of natural uranium (to produce about 25 tonnes of enriched uranium).
Consumption of a 3000MWth (~1000MWe) pressurized water reactor (12-month fuel cycle)
It is an illustrative example, and the following data do not correspond to any reactor design.
- A typical reactor may contain about 100 tonnes of enriched uranium (i.e., about 113 tonnes of uranium dioxide).
- This fuel is loaded within, for example, 157 fuel assemblies composed of over 45,000 fuel rods.
- A common fuel assembly contains energy for approximately 4 years of operation at full power.
- Therefore about one-quarter of the core is yearly removed from the spent fuel pool (i.e., about 40 fuel assemblies). At the same time, the remainder is rearranged to a location in the core better suited to its remaining level of enrichment (see Power Distribution).
- This reactor’s annual natural uranium consumption is about 250 tonnes of natural uranium (to produce about 25 tonnes of enriched uranium).
- This reactor’s annual enriched uranium consumption is about 25 tonnes of enriched uranium.