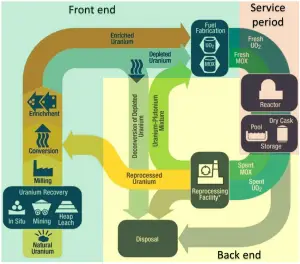
Uranium milling is a series of mechanical and chemical processes that process extracted uranium ore and produces dry powder-form material consisting of natural uranium called “yellowcake” (chemically U3O8) because of its yellowish color. Uranium ores are normally processed by grinding the ore materials to uniform particle size and then treating the ore to extract the uranium by chemical leaching.
A conventional uranium mill is a chemical plant, usually built near the uranium mine. Uranium milling extracts uranium using the following process:
- Milling. Trucks deliver uranium ore to the mill, which is crushed into smaller particles before extraction (or leaching). Due to the very low concentration of uranium in the rock, immense amounts of rock must be moved and processed to get a few kilograms of natural uranium. This results in enormous heaps. Due to high amounts of material, milling is generally carried out close to a uranium mine.
- Leaching. After milling, a fine slurry is leached in sulfuric acid (or sometimes a strong alkaline solution) to allow the separation of uranium from the waste rock. It is then recovered from the solution and precipitated as uranium oxide (U3O8) concentrate. After drying and usually heating, a yellow-brownish powder, yellowcake, is packed in 200-liter drums as a concentrate. In addition to extracting 90 to 95 percent of the uranium from the ore, the leaching agent also extracts several other “heavy metal” constituents, including molybdenum, vanadium, selenium, iron, lead, and arsenic.)
- Further processing. Yellowcake is then refined to obtain almost pure uranium in the form of triuranium octoxide (U3O8). Pure U3O8 is then used to prepare uranium fuel for nuclear reactors, for which triuranium octoxide powder may be sintered into fuel pellets (UO2) for use in fuel rods for pressurized heavy-water reactors. For other systems that use enriched uranium, uranium conversion and enrichment must follow this process.
The remainder of the ore, containing most of the radioactivity and nearly all the rock material, becomes uranium tailings, which are emplaced in engineered facilities near the mine (often in a mined-out pit). It must be noted that uranium tailings contain almost all the descendants of radioactive cascades that have been in radioactive equilibrium for millions of years. For example, pure uranium-238, from the uranium series, is weakly radioactive (proportional to its long half-life). Still, uranium ore is about 13 times more radioactive than the pure uranium-238 metal because of its daughter isotopes (e.g., radon, radium, etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain, they also generate radon, a heavy, inert, naturally occurring radioactive gas. At this place, we are discussing natural facts, and it must be noted that the decay heat of uranium and its decay products (e.g., radon, radium, etc.) contributes to the heating of Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid. But uranium tailings need to be isolated from the environment because they are concentrated and contain these long-lived radioactive materials from the radioactive decay chain.
Consumption of a 3000MWth (~1000MWe) pressurized water reactor (12-month fuel cycle)
It is an illustrative example, and the following data do not correspond to any reactor design.
- A typical reactor may contain about 100 tonnes of enriched uranium (i.e., about 113 tonnes of uranium dioxide).
- This fuel is loaded within, for example, 157 fuel assemblies composed of over 45,000 fuel rods.
- A common fuel assembly contains energy for approximately 4 years of operation at full power.
- Therefore about one-quarter of the core is yearly removed from the spent fuel pool (i.e., about 40 fuel assemblies). At the same time, the remainder is rearranged to a location in the core better suited to its remaining level of enrichment (see Power Distribution).
The annual natural uranium consumption of this reactor is about 250 tonnes of natural uranium (to produce about 25 tonnes of enriched uranium).