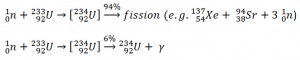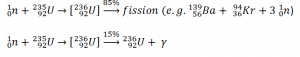What is Thorium
Thorium is a naturally-occurring chemical element with atomic number 90, which means there are 90 protons and 90 electrons in the atomic structure. The chemical symbol for thorium is Th. Thorium was discovered in 1828 by Norwegian mineralogist Morten Thrane Esmark. Joens Jakob Berzelius, the Swedish chemist, named it after Thor, the Norse god of thunder.
Thorium is a naturally-occurring element, and it is estimated to be about three times more abundant than uranium. Thorium is commonly found in monazite sands (rare earth metals containing phosphate minerals).
Thorium has 6 naturally occurring isotopes. All of these isotopes are unstable (radioactive), but only 232Th is relatively stable with a half-life of 14 billion years, which is comparable to the age of the Earth (~4.5×109 years). Isotope 232Th belongs to primordial nuclides, and natural thorium consists primarily of isotope 232Th. Other isotopes (230Th, 229Th, 228Th, 234Th, and 227Th) occur in nature as trace radioisotopes, which originate from the decay of 232Th, 235U, and 238U.
Thorium 232
Thorium 232, which alone makes up nearly all natural thorium, is the most common isotope of thorium in nature. This isotope has the longest half-life (1.4 x 1010 years) of all isotopes with more than 83 protons. In fact, its half-life is considerably longer than the age of Earth. Therefore 232Th belongs to primordial nuclides.
232Th decays via alpha decay into 228Ra. 232Th occasionally decays by spontaneous fission with a very low probability of 1.1 x 10-9 %.
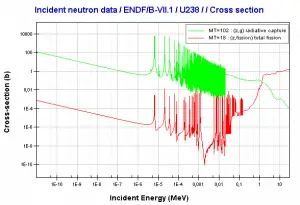
232Th is a fertile isotope. 232Th is not capable of undergoing a fission reaction after absorbing a thermal neutron. On the other hand, 232Th can be fissioned by fast neutron with energy higher than >1MeV. 232Th does not also meet the alternative requirement to fissile materials. 232Th cannot sustain a nuclear fission chain reaction because too many neutrons produced by fission of 232Th have lower energies than the original neutron.
Isotope 232Th is key material in the thorium fuel cycle. Radiative capture of a neutron leads to the formation of fissile 233U. This process is called nuclear fuel breeding.
What is Uranium
Uranium is a naturally-occurring chemical element with atomic number 92, which means there are 92 protons and 92 electrons in the atomic structure. The chemical symbol for uranium is U. Uranium was discovered in 1789 by Martin Klaproth in the mineral called pitchblende (uraninite). He named the newly discovered element after the planet Uranus, discovered eight years earlier. It was first isolated as a metal in 1841 by Eugene-Melchior Peligot. Henri Becquerel discovered uranium to be radioactive in 1896. He discovered that uranium minerals could expose a photographic plate through another material. In fact, he was the first to discover the process of radioactivity.
Uranium is commonly found at low levels (a few ppm – parts per million) in all rocks, soil, water, plants, and animals (including humans). Uranium also occurs in seawater and can be recovered from ocean water. Significant concentrations of uranium occur in some substances such as uraninite (the most common uranium ore), phosphate rock deposits, and other minerals.
Natural uranium consists primarily of isotope 238U(99.28%); therefore, the atomic mass of the uranium element is close to the atomic mass of 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). The abundance of isotopes in nature is caused by the difference in the half-life. All three naturally-occurring isotopes of uranium (238U, 235U, and 234U) are unstable. On the other hand, these isotopes (except 234U) belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.5×109 years for 238U).
In nuclear reactors, we have to consider three artificial isotopes, 236U, 233U, and 232U. These are produced by transmutation in nuclear reactors from 235U and 232Th.

Thorium vs. Uranium – Fuel Breeding
232Th is the predominant isotope of natural thorium. If this fertile material is loaded in the nuclear reactor, the nuclei of 232Th absorb a neutron and become nuclei of 233Th. The half-life of 233Th is approximately 21.8 minutes. 233Th decays (negative beta decay) to 233Pa (protactinium), whose half-life is 26.97 days. 233Pa decays (negative beta decay) to 233U, that is very good fissile material. On the other hand proposed reactor designs must attempt to physically isolate the protactinium from further neutron capture before beta decay can occur.
Neutron capture may also be used to create fissile 239Pu from 238U, the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.
Transmutation of transuranium elements (i.e., the chemical elements with atomic numbers greater than 92 ) such as the isotopes of plutonium has the potential to help solve some problems posed by the management of radioactive waste by reducing the proportion of long-lived isotopes it contains.
Conversion Factors
A quantity that characterizes this conversion of fertile into fissile material is known as the conversion factor. The conversion factor is defined as the ratio of fissile material created to fissile material consumed either by fission or absorption. If the ratio is greater than one, it is often referred to as the breeding ratio, for then the reactor is creating more fissile material than it is consuming.
When C is unity, one new atom is produced per one atom consumed. It seems fertile material can be converted in the reactor indefinitely without adding new fuel. Still, in real reactors, the content of fertile thorium 232 also decreases, and fission products with significant absorption cross-section accumulate in the fuel as fuel burnup increases.
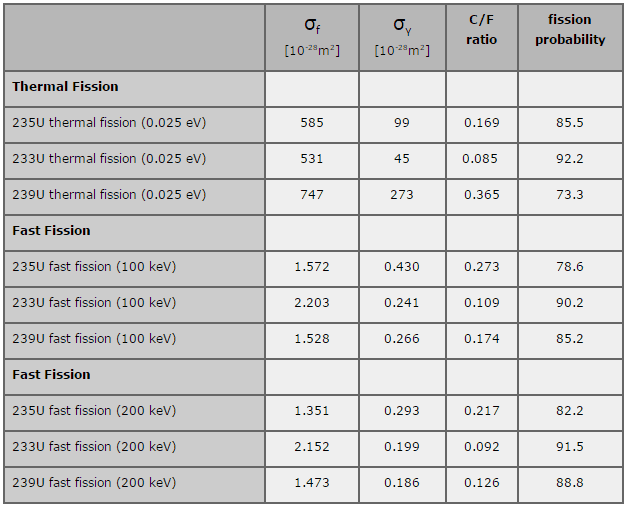
The fertile-to-fissile conversion characteristics depend on the fuel cycle and the neutron energy spectrum. For a thermal neutron spectrum (E < 1 eV) and the uranium fuel cycle, fuel breeding (C>1) is not feasible, although η for both isotopes is greater than 2. This is due to the fact η is not large enough to compensate for the neutron leakage and its parasitic capture.
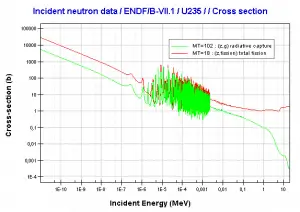
For a thermal neutron spectrum (E < 1 eV) and the thorium fuel cycle, the situation is considerably better. Due to very low capture-to-fission ratio, the reproduction factor for uranium 233 is about η = 2.25. From this point of view is thorium fuel cycle is promising and a thermal reactor of this type could successfully be made to bred.
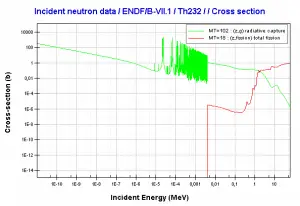
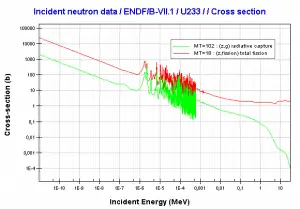
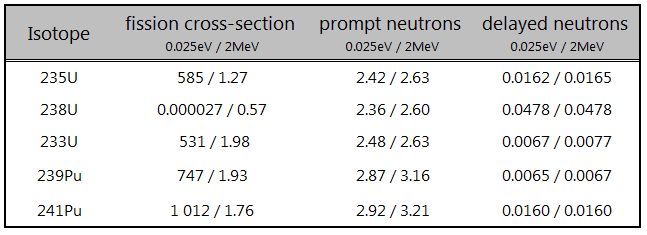
Thorium vs Uranium – Cross-sections
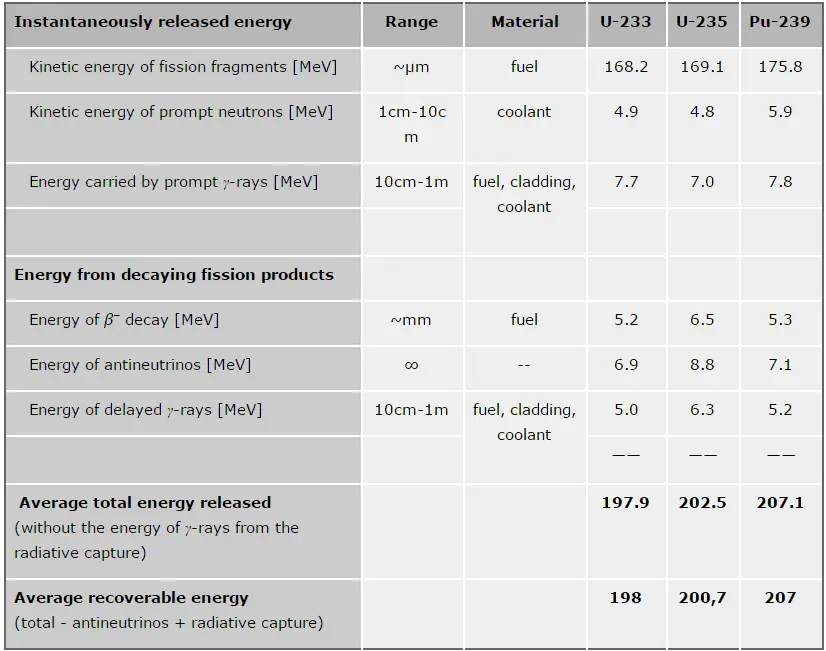
Thorium vs. Uranium – Energy Release per Fission
In general, nuclear fission results in the release of enormous quantities of energy. The amount of energy depends strongly on the nucleus to be fissioned and also depends strongly on the kinetic energy of an incident neutron. It is necessary to be able to precisely identify the individual components of this energy to calculate the power of a reactor. At first, it is important to distinguish between the total energy released and the energy that can be recovered in a reactor.
The total energy released in fission can be calculated from binding energies of the initial target nucleus to be fissioned and binding energies of fission products. But not all the total energy can be recovered in a reactor. For example, about 10 MeV is released in the form of neutrinos (in fact, antineutrinos). Since the neutrinos are weakly interacting (with an extremely low cross-section of any interaction), they do not contribute to the energy that can be recovered in a reactor.
Thorium vs. Uranium – Delayed Neutrons
Despite the fact, the number of delayed neutrons per fission neutron is quite small (typically below 1%) and thus does not contribute significantly to the power generation, they play a crucial role in reactor control. They are essential from the point of view of reactor kinetics and reactor safety. Their presence completely changes the dynamic time response of a reactor to some reactivity change, making it controllable by control systems such as the control rods.
Delayed neutrons allow to operate a reactor in a prompt subcritical, delayed critical condition. All power reactors are designed to operate in delayed critical conditions and are provided with safety systems to prevent them from ever achieving prompt criticality.