What is Thorium
Thorium is a naturally occurring chemical element with the atomic number 90, which means there are 90 protons and 90 electrons in the atomic structure. The chemical symbol for thorium is Th. Thorium was discovered in 1828 by Norwegian mineralogist Morten Thrane Esmark. Joens Jakob Berzelius, the Swedish chemist, named it after Thor, the Norse god of thunder.
Thorium is a naturally occurring element, and it is estimated to be about three times more abundant than uranium. Thorium is commonly found in monazite sands (rare earth metals containing phosphate minerals).
Thorium has 6 naturally occurring isotopes. All of these isotopes are unstable (radioactive), but only 232Th is relatively stable with half-life of 14 billion years, which is comparable to the age of the Earth (~4.5×109 years). Isotope 232Th belongs to primordial nuclides and natural thorium consists primarily of isotope 232Th. Other isotopes (230Th, 229Th, 228Th, 234Th and 227Th) occur in nature as trace radioisotopes, which originate from decay of 232Th, 235U and 238U.
Thorium 232
Thorium 232, which alone makes up nearly all natural thorium, is the most common isotope of thorium in nature. This isotope has the longest half-life (1.4 x 1010 years) of all isotopes with more than 83 protons. Its half-life is considerably longer than the age of the earth. Therefore 232Th belongs to primordial nuclides.
232Th decays via alpha decay into 228Ra. 232Th occasionally decays by spontaneous fission with a very low probability of 1.1 x 10-9 %.
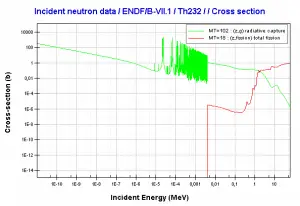
232Th is a fertile isotope. 232Th is not capable of undergoing a fission reaction after absorbing a thermal neutron. On the other hand, 232Th can be fissioned by fast neutron with energy higher than >1MeV. 232Th does not also meet alternative requirements to fissile materials. 232Th cannot sustain a nuclear fission chain reaction because too many neutrons produced by fission of 232Th have lower energies than the original neutron.
Isotope 232Th is key material in the thorium fuel cycle. Radiative capture of a neutron leads to the formation of fissile 233U. This process is called nuclear fuel breeding.