Decay mode and Half-lifes of Isotopes of Uranium
Natural uranium consists primarily of isotope 238U (99.28%). Therefore the atomic mass of the uranium element is close to the atomic mass of the 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). Differences in the half-lives cause the abundance of isotopes in nature. All three naturally occurring isotopes of uranium (238U, 235U, and 234U) are unstable. On the other hand, these isotopes (except 234U) belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.5×109 years for 238U). In nuclear reactors, we have to consider three artificial isotopes, 236U, 233U, and 232U. These are produced by transmutation in nuclear reactors from 235U and 232Th.
The main isotopes, which have to be considered in the fuel cycle of all commercial light water reactors, are:Naturally-occurring isotopes
- 238U. 238U decays via alpha decay to 234Th with a half-life of ~4.5×109 years. 238U occasionally decays by spontaneous fission with the probability of 0.000055%. Its specific activity is very low ~3.4×10-7 Ci/g.
- 235U. 235U decays via alpha decay (thorium-231) into 231Pa with a half-life of ~7×108 years. 235U occasionally decays by spontaneous fission with a very low probability of 0.0000000072%. Its specific activity is very low ~2.2×10-6 Ci/g.
- 234U. 234U decays via alpha decay to 230Th with a half-life of 246 000 years. 234U occasionally decays by spontaneous fission with a very low probability of 0.0000000017%. Its specific activity is much higher ~0.0063 Ci/g.
Artificial isotopes
- 233U. 233U decays via alpha decay into 229Th with a half-life of 159 200 years. 233U occasionally decays by spontaneous fission with a very low probability of 0.000000006%. Its specific activity is ~0.0098 Ci/g.
- 236U. 236U decays via alpha decay to 232Th with a half-life of ~2.3×107 years. 236U occasionally decays by spontaneous fission with a very low probability of 0.00000009%. Its specific activity is ~6.5×10-5 Ci/g.
- 232U. 232U decays via alpha decay to 228Th with a half-life of 68.9 years. 232U very rarely decays by spontaneous fission. Its specific activity is very high, ~22 Ci/g, and its decay chain produces very penetrating gamma rays.
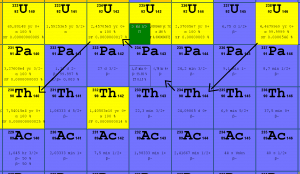 Uranium 238 decay. Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Uranium 238 decay. Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library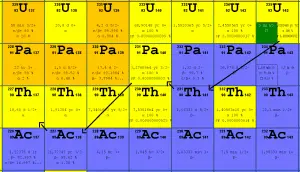 Uranium 235 decay. Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Uranium 235 decay. Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
| Isotope | Specific Activity |
|---|---|
| Uranium 232 | 22 Ci/g |
| Uranium 233 | 0.0098 Ci/g |
| Uranium 234 | 0.0063 Ci/g |
| Uranium 236 | 0.000065 Ci/g |
| Uranium 235 | 0.0000022 Ci/g |
| Uranium 238 | 0.00000034 Ci/g |
Table of specific activities of isotopes of uranium.