Natural Uranium
Natural uranium refers to uranium with the same isotopic ratio as found in nature. It consists primarily of isotope 238U (99.28%). Therefore the atomic mass of the uranium element is close to the atomic mass of the 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). Differences in the half-lives cause the abundance of isotopes in nature. All three naturally occurring isotopes of uranium (238U, 235U, and 234U) are unstable. On the other hand, these isotopes (except 234U) belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.5×109 years for 238U).
Natural uranium is commonly found at low levels (a few ppm – parts per million) in all rocks, soil, water, plants, and animals (including humans). Uranium also occurs in seawater and can be recovered from ocean water. Significant concentrations of uranium occur in some substances such as uraninite (the most common uranium ore), phosphate rock deposits, and other minerals.
Uranium in the Environment
All three naturally occurring uranium isotopes (238U, 235U, and 234U) have a very long half-life (e.g.,, 4.47×109 years for 238U). Because of this very long half-life, uranium is weakly radioactive and contributes to low natural background radiation levels in the environment. These isotopes are alpha radioactive (emitting alpha particles), but they can also rarely spontaneously fission.
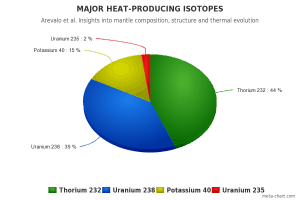
All naturally occurring isotopes belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.54×109 years). Uranium has the second-highest atomic mass of these primordial nuclides, lighter only than plutonium. Moreover, the decay heat of uranium and its decay products (e.g.,, radon, radium, etc.) contributes to heating the Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid.
Natural uranium consumption in a nuclear reactor
Since natural uranium contains only 0.71% of fissile isotope 235U and most current power reactors require enriched uranium, this natural uranium must be enriched. The level of enrichment required depends on specific reactor design (e.g.,, PWRs and BWRs require 3% – 5% of 235U) and specific requirements of the nuclear power plant operator. Without required enrichment, these reactors cannot initiate and sustain a nuclear chain reaction for such a long period as 12 months (or more).
The enrichment process separates gaseous uranium hexafluoride into two streams, one being enriched to the required level and known as low-enriched uranium, the other stream is progressively depleted in U-235 and is called ‘tails’, or simply depleted uranium. Typically, to produce 1 kg of enriched uranium with 5% of 235U, about 10 kg of natural uranium is required with a byproduct of about 9 kg of depleted uranium. Therefore annual natural uranium consumption of 3000MWth reactor is about 250 tonnes of natural uranium (to produce of about 25 tonnes of enriched uranium).