Uranium 234
Uranium 234, which alone constitutes only 0.0054% (54 parts per million) of natural uranium, is the last naturally occurring isotope of uranium. This isotope has a half-life of only 2.46×105 years, and therefore it does not belong to primordial nuclides (unlike 235U and 238U). On the other hand, this isotope is still present in the Earth’s crust, but this is due to the fact
234U is an indirect decay product of 238U. 238U decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U. 234U decays via alpha decay into 230Th, except very small fraction (on the order of ppm) of nuclei which decays by spontaneous fission.
In a natural sample of uranium, these nuclei are present in the unalterable proportions of the radioactive equilibrium of the 238U filiation at a ratio of one atom of 234U for about 18 500 nuclei 238U. As a result of this equilibrium, these two isotopes (238U and 234U) contribute equally to the radioactivity of natural uranium.
Since uranium enrichment separates light isotopes from heavy isotopes, uranium enrichment also results in the enrichment of 234U. Therefore enriched uranium also contains more isotope 234U than natural uranium. On the other hand, enrichment tails or also depleted uranium contain much less than 234U. In nuclear reactors, which use enriched uranium as a fuel, such increased content of 234U is acceptable. Undesirable concentrations may be reached when using reprocessed uranium because spent nuclear fuel may contain a much higher concentration of about 0.01% of 234U.
234U is a non-fissile isotope, and it is not capable of undergoing a fission reaction after absorbing a thermal neutron. 234U belongs to the group of fertile isotopes. Radiative capture of a neutron leads to the formation of fissile 235U, similarly to 238U, which radiative capture leads to the formation of fissile 239Pu. The radiative capture cross-section for 234U is about 100 barns for thermal neutrons. Therefore 234U is converted to 235U more easily and therefore at a greater rate than 238U is to 239Pu (nuclei of 238U have a much smaller cross-section of 2 barns). On the other hand, the effect of 235U breeding is almost insignificant compared to the 239Pu breeding.
See also: Comparison of cross-sections
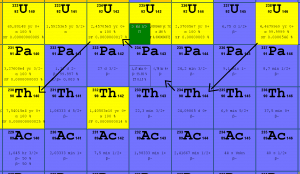 Uranium 238 decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U.
Uranium 238 decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U.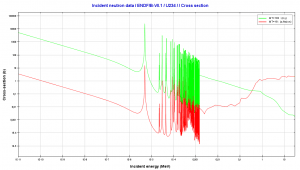 Uranium 234. Comparison of total fission cross-section and cross-section for radiative capture.
Uranium 234. Comparison of total fission cross-section and cross-section for radiative capture.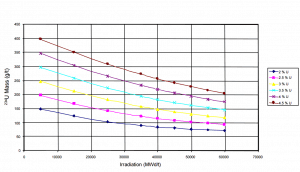 Residual 234U content as function of burnup level of PWR fuel.
Residual 234U content as function of burnup level of PWR fuel.Source: IAEA-TECDOC-1529 Management of Reprocessed Uranium