Uranium 238 has the longest half-life (4.47×109 years), and therefore its abundance is so high.
Uranium 238 is a fissionable isotope but is not a fissile isotope.
238U belongs to primordial nuclides because its half-life is comparable to the age of the Earth (~4.5×109 years). For its very long half-life, it is still present in the Earth’s crust.238U decays via alpha decay (by way of thorium 234 and protactinium 234) into 234U. 238U occasionally decays by spontaneous fission with the probability of 0.000055%.
238U is a fissionable isotope but is not a fissile isotope. 238U is not capable of undergoing a fission reaction after absorbing a thermal neutron. On the other hand, 238U can be fissioned by fast neutron with energy higher than >1MeV. 238U does not also meet the alternative requirement to fissile materials. 238U cannot sustain a nuclear fission chain reaction because too many neutrons produced by the fission of 238U have lower energies than the original neutron.
238U also belongs to the group of fertile isotopes. Radiative capture of a neutron leads to the formation of fissile 239Pu. This is how 238U contributes to the operation of nuclear reactors and the production of electricity through this plutonium. For example, at a burnup of 40GWd/tU, about 40% of the total energy released comes from bred plutonium. This corresponds to a breeding ratio for this fuel burnup of about 0.4 to 0.5. This effect extends the cycle length for such fuels to sometimes nearly twice what it would be otherwise.
See also: Comparison of cross-sections.
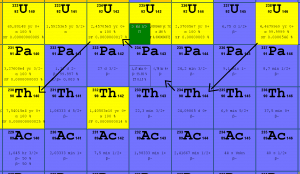 Uranium 238 decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U.
Uranium 238 decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U.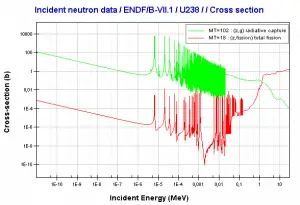 Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.
Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.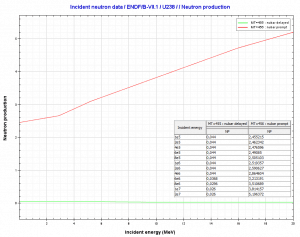 Neutron production per one fast fission of uranium 238.
Neutron production per one fast fission of uranium 238.