Article Summary & FAQs
What is liquid drop model of nucleus?
In nuclear physics, the liquid drop model of the nucleus describes forces in atomic nuclei as if a tiny liquid drop formed the atomic nucleus. But in this nuclear scale, the fluid is made of nucleons (protons and neutrons). The liquid drop model considers that the forces on the nucleons on the surface are different from those on nucleons on the interior, where other attracting nucleons completely surround them. This is similar to taking into account surface tension as a contributor to the energy of a tiny liquid drop.
Key Facts
- Scattering experiments suggest that nuclei have approximately constant density.
- Nuclei have their volume and surface, where forces act differently.
- In the ground state, the nucleus is spherical.
- If sufficient kinetic or binding energy is added, this spherical nucleus may be distorted into a dumbbell shape and then maybe split into two fragments.
- The Weizsaecker formula is an empirically refined form of the liquid drop model for the binding energy of nuclei. It has the following terms:
- Volume term
- Surface term
- Asymmetry term
- Pairing term
- Using the Weizsaecker formula, the binding energies and also masses of atomic nuclei can be derived. Therefore, we can also derive the energy released per fission.
Liquid Drop Model of Nucleus
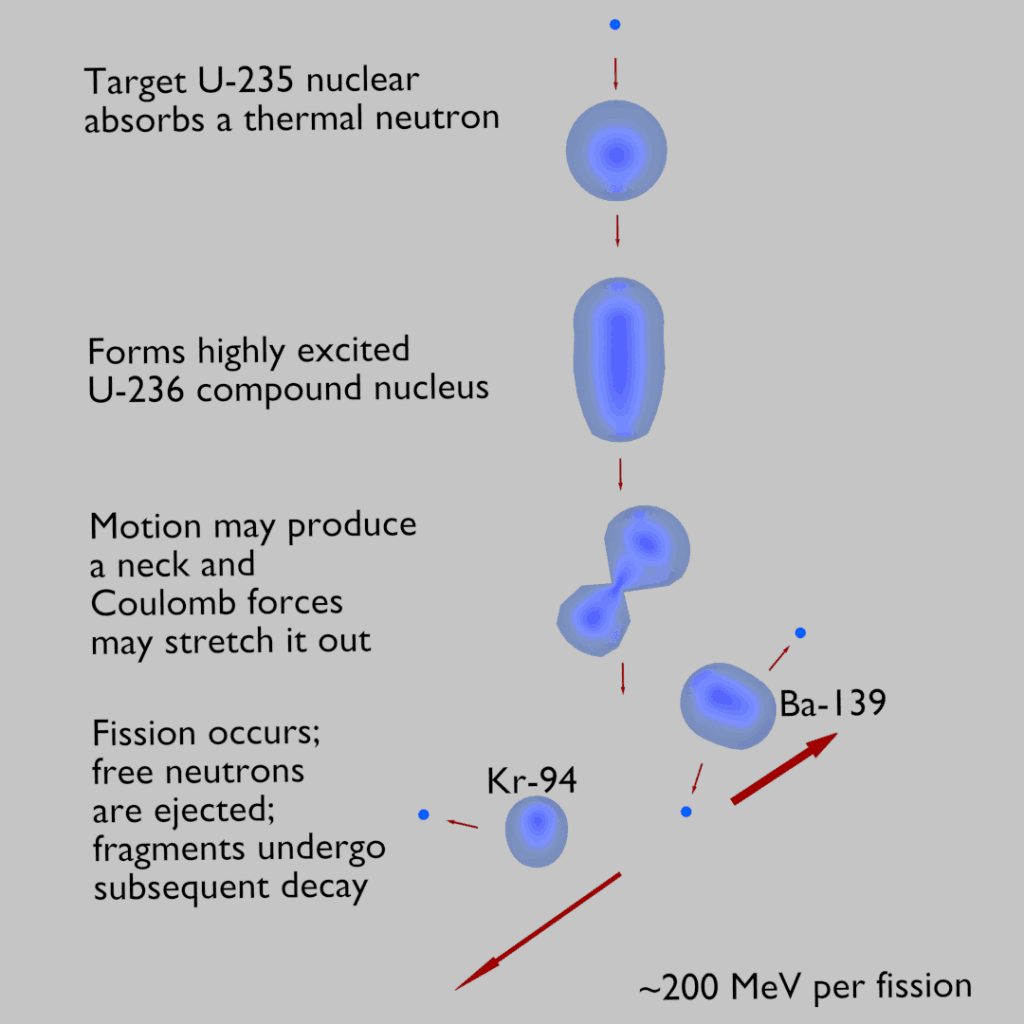 One of the first models that could describe the behavior of the nuclear binding energies and therefore of nuclear masses was the mass formula of von Weizsaecker (also called the semi-empirical mass formula – SEMF) published in 1935 by German physicist Carl Friedrich von Weizsäcker. This theory is based on the liquid drop model proposed by George Gamow.
One of the first models that could describe the behavior of the nuclear binding energies and therefore of nuclear masses was the mass formula of von Weizsaecker (also called the semi-empirical mass formula – SEMF) published in 1935 by German physicist Carl Friedrich von Weizsäcker. This theory is based on the liquid drop model proposed by George Gamow.
According to this model, the atomic nucleus behaves like the molecules in a drop of liquid. But in this nuclear scale, the fluid is made of nucleons (protons and neutrons), held together by the strong nuclear force. The liquid drop model of the nucleus considers that the nuclear forces on the nucleons on the surface are different from those on nucleons in the interior of the nucleus. Other attractive nucleons completely surround the interior nucleons. Here is the analogy with the forces that form a drop of liquid.
In the ground state, the nucleus is spherical. If sufficient kinetic or binding energy is added, this spherical nucleus may be distorted into a dumbbell shape and then maybe split into two fragments. Since these fragments are more stable, the splitting of such heavy nuclei must be accompanied by energy release. This model does not explain all the properties of the atomic nucleus but does explain the predicted nuclear binding energies.
The nuclear binding energy as a function of the mass number A and the number of protons Z based on the liquid drop model can be written as: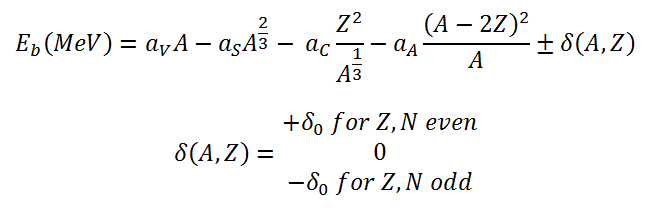 This formula is called the Weizsaecker Formula (or the semi-empirical mass formula). The physical meaning of this equation can be discussed term by term.
This formula is called the Weizsaecker Formula (or the semi-empirical mass formula). The physical meaning of this equation can be discussed term by term.
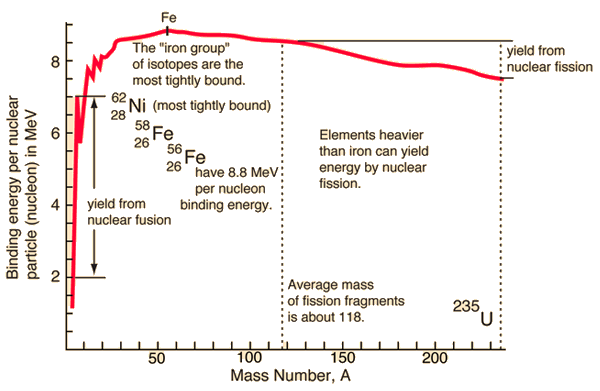
Source: hyperphysics.phy-astr.gsu.edu
With the aid of the Weizsaecker formula, the binding energy can be calculated very well for nearly all isotopes. This formula provides a good fit for heavier nuclei. For light nuclei, especially for 4He, it provides a poor fit. The main reason is the formula does not consider the internal shell structure of the nucleus.
The coefficients aV, aS, aC, aA, and aP must be known to calculate the binding energy. The coefficients have units of megaelectronvolts (MeV) and are calculated by fitting to experimentally measured masses of nuclei. They usually vary depending on the fitting methodology. According to ROHLF, J. W., Modern Physics from α to Z0, Wiley, 1994., the coefficients in the equation are the following: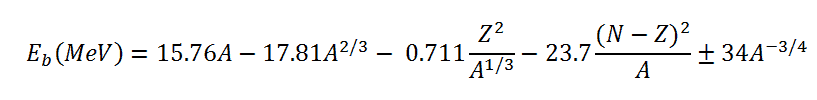 Using the Weizsaecker formula. Also, the mass of an atomic nucleus can be derived and is given by:
Using the Weizsaecker formula. Also, the mass of an atomic nucleus can be derived and is given by:
m = Z.mp +N.mn -Eb/c2
where mp and mn are the rest mass of a proton and a neutron, respectively, and Eb is the nuclear binding energy of the nucleus. From the nuclear binding energy curve and the table, it can be seen that, in the case of splitting a 235U nucleus into two parts, the binding energy of the fragments (A ≈ 120) together is larger than that of the original 235U nucleus.
According to the Weizsaecker formula, the total energy released for such reaction will be approximately 235 x (8.5 – 7.6) ≈ 200 MeV.
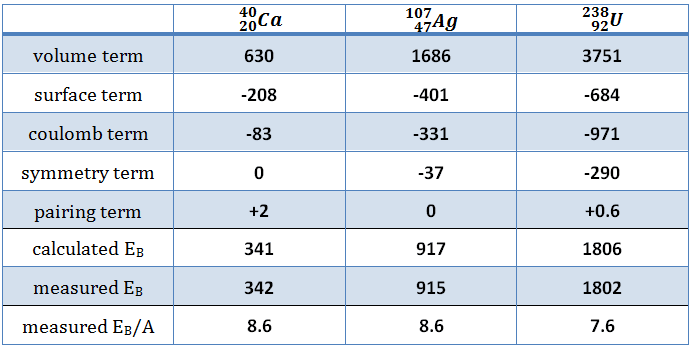 Table of binding energies fo some nuclides. Calculated according to the semi-empirical mass formula.
Table of binding energies fo some nuclides. Calculated according to the semi-empirical mass formula.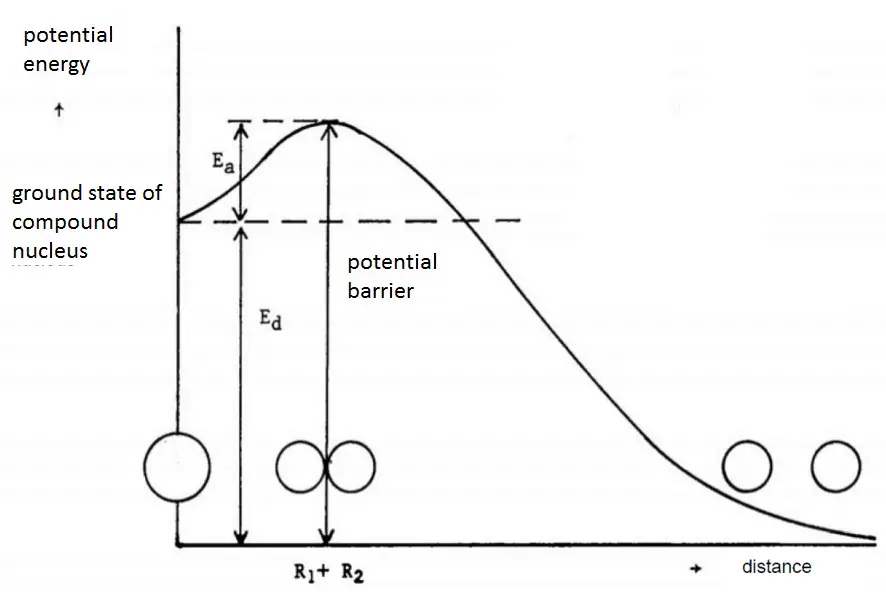 The minimum excitation energy required for fission to occur is known as the critical energy (Ecrit) or threshold energy.
The minimum excitation energy required for fission to occur is known as the critical energy (Ecrit) or threshold energy.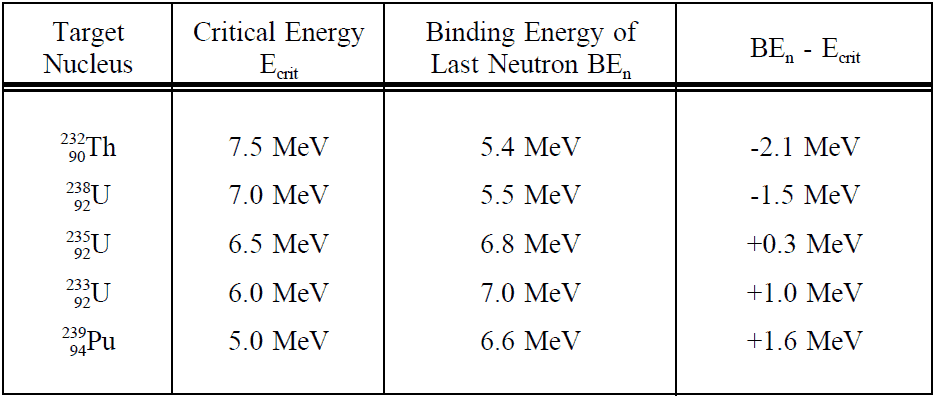 This table shows critical energies compared to binding energies of the last neutron of a number of nuclei.
This table shows critical energies compared to binding energies of the last neutron of a number of nuclei.