Quiz-summary
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
|
You must specify a text. |
Test your Knowledge – Nuclear Reactions
With our simple quizzes, you can test your knowledge.
It is intuitive: start quiz and answer questions.
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 20 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- Nuclear Reactions 0%
-
Here, you can find answers on all the questions:
Share your results on Social Media.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
These nuclear reactions are fast and involve a single-nucleon interaction. The interaction time must be very short (~10-22 s). Incident particles interact on the surface of a target nucleus rather than in the volume of a target nucleus.
This is typical for:
-
Question 2 of 20
2. Question
A projectile and a target nucleus are within the range of nuclear forces for the time allowing for a large number of interactions between nucleons.
This is typical for:
-
Question 3 of 20
3. Question
Products of these reactions are distributed near isotropically in angle (the nucleus loses memory of how it was created – the Bohr’s hypothesis of independence). The mode of decay of the nucleus do not depend on the way the nucleus is formed.
This is typical for:
-
Question 4 of 20
4. Question
Typical direct nuclear reaction is:
-
Question 5 of 20
5. Question
Typical compound nucleus reaction is:
-
Question 6 of 20
6. Question
One the following statements is not typical for nuclear resonances?
Correct
See: Compound Nucleus Reactions
Explanation: The observation of resonances is by no means restricted to neutron-nuclear reactions.
Incorrect
See: Compound Nucleus Reactions
Explanation: The observation of resonances is by no means restricted to neutron-nuclear reactions.
-
Question 7 of 20
7. Question
Raising the temperature of PWR fuel causes the fuel nuclei to vibrate more rapidly within their lattice structures. This causes an increase in resonance absorption of neutrons.
Correct
See: Neutron Absorption
This phenomenon is known as the Doppler broadening of resonances, which is very important phenomenon, that improves reactor stability.
Incorrect
See: Neutron Absorption
This phenomenon is known as the Doppler broadening of resonances, which is very important phenomenon, that improves reactor stability.
-
Question 8 of 20
8. Question
The reaction 40Ca (α, α’)40mCa belongs to:
-
Question 9 of 20
9. Question
One of the following reactions is not a mechanism of neutron elastic scattering reactions:
Correct
See: Elastic Scattering Reactions
The neutron elastic scattering can occur by way of two interaction mechanisms:
- Potential scattering. In potential scattering, the neutron and the nucleus interact without neutron absorption and the formation of a compound nucleus. In fact, the incident neutron does not necessarily have to “touch” the nucleus and the neutron is scattered by the short range nuclear forces when it approaches close enough to the nucleus. Potential scattering occurs with incident neutrons that have an energy of up to about 1 MeV. It may be modeled as a billiard ball collision between a neutron and a nucleus.
- Compound-elastic scattering. In some cases, if the kinetic energy of an incident neutron just right to form a resonance, the neutron may be absorbed and a compound nucleus may be formed. This interaction is more unusual and is also known as resonance elastic scattering. Due to formation of the compound nucleus, initial and final neutron are not the same.
Incorrect
See: Elastic Scattering Reactions
The neutron elastic scattering can occur by way of two interaction mechanisms:
- Potential scattering. In potential scattering, the neutron and the nucleus interact without neutron absorption and the formation of a compound nucleus. In fact, the incident neutron does not necessarily have to “touch” the nucleus and the neutron is scattered by the short range nuclear forces when it approaches close enough to the nucleus. Potential scattering occurs with incident neutrons that have an energy of up to about 1 MeV. It may be modeled as a billiard ball collision between a neutron and a nucleus.
- Compound-elastic scattering. In some cases, if the kinetic energy of an incident neutron just right to form a resonance, the neutron may be absorbed and a compound nucleus may be formed. This interaction is more unusual and is also known as resonance elastic scattering. Due to formation of the compound nucleus, initial and final neutron are not the same.
-
Question 10 of 20
10. Question
Elastic scattering (cross-section) of slow neutrons by molecules is greater than by free nuclei.
Correct
See: Elastic Scattering Reactions
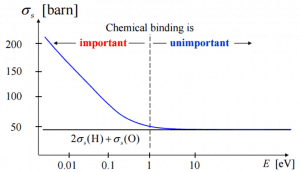
If the kinetic energy of an incident neutron is large compared with the chemical binding energy of the atoms in a molecule, the chemical bound can be ignored.
If the kinetic energy of an incident neutron is of the order or less than the chemical binding energy, the cross-section of the molecule is not equal to the sum of cross-sections of its individual nuclei.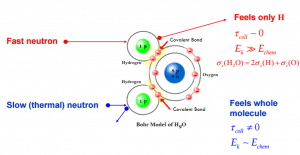
Scattering of slow neutrons by molecules is greater than by free nuclei. Incorrect
See: Elastic Scattering Reactions

If the kinetic energy of an incident neutron is large compared with the chemical binding energy of the atoms in a molecule, the chemical bound can be ignored.
If the kinetic energy of an incident neutron is of the order or less than the chemical binding energy, the cross-section of the molecule is not equal to the sum of cross-sections of its individual nuclei.
Scattering of slow neutrons by molecules is greater than by free nuclei. -
Question 11 of 20
11. Question
During an inelastic scattering the neutron can be absorbed and then re-emitted. This reaction belongs to direct reactions.
Incorrect
See: Inelastic Scattering Reactions
Explanation: The reaction occurs via compound nucleus.
-
Question 12 of 20
12. Question
One the following statements is not typical for neutron inelastic reactions?
Correct
See: Inelastic Scattering Reactions
Explanation: For hydrogen nucleus, inelastic scattering does not occur, because it does not have excited states.
Incorrect
See: Inelastic Scattering Reactions
Explanation: For hydrogen nucleus, inelastic scattering does not occur, because it does not have excited states.
-
Question 13 of 20
13. Question
The most important neutron absorption reactions are divided by the exit channel into two types of reactions. One the following reactions is not an absorption reaction.
-
Question 14 of 20
14. Question
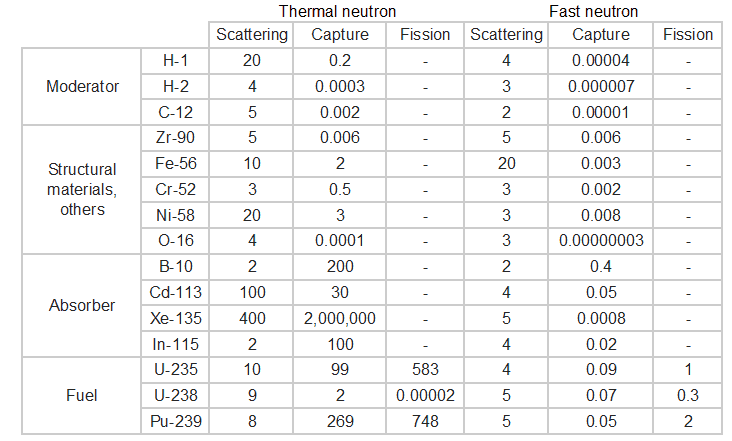
Sort the following isotopes according to the radiative capture cross-section for thermal neutrons from highest to lowest cross-section.
-
Xe-135
-
Gd-157
-
U-235
-
U-238
-
H-1 (Hydrogen)
-
H-2 (Deuterium)
-
-
Question 15 of 20
15. Question
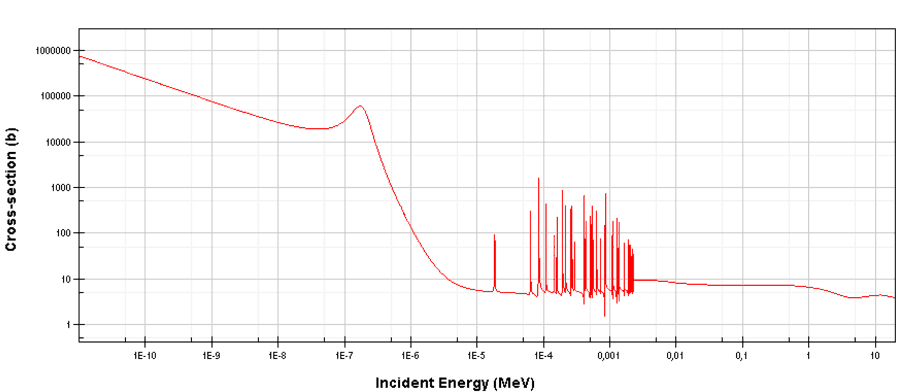
For which neutron absorber is the following chart (cross-sections) typical (see the cut-off energy ~0.5 eV)?
Correct
See: Cadmium
There is a cadmium cut-off energy (Cadmium edge) in the absorption cross-section. Only neutrons of kinetic energy below the cadmium cut-off energy (~0.5 eV) are strongly absorbed by 113Cd. Therefore cadmium is widely used to absorb thermal neutrons in a thermal neutron filters.
Incorrect
See: Cadmium
There is a cadmium cut-off energy (Cadmium edge) in the absorption cross-section. Only neutrons of kinetic energy below the cadmium cut-off energy (~0.5 eV) are strongly absorbed by 113Cd. Therefore cadmium is widely used to absorb thermal neutrons in a thermal neutron filters.
-
Question 16 of 20
16. Question
What is not typical for (γ, n) reactions (production of photoneutrons)?
Correct
See: Photoneutrons
Explanation: Photoneutrons are usually major source in a reactor and ensure sufficient neutron flux on source range detectors when reactor is subcritical in long term shutdown.
Incorrect
See: Photoneutrons
Explanation: Photoneutrons are usually major source in a reactor and ensure sufficient neutron flux on source range detectors when reactor is subcritical in long term shutdown.
-
Question 17 of 20
17. Question
The most important neutron nuclear reactions in nuclear reactor physics are reactions of thermal neutrons with boron nuclei (rather with 10B nuclei). In nuclear industry boron is commonly used as a neutron absorber and a neutron converter.
Which type of reaction is used in neutron absorbers based on boron (n + 10B)?
Correct
See: Boron 10
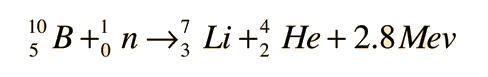
This equation describes neutron capture in the boron, which is diluted in the coolant. Boric acid is used in nuclear power plants as a long-term compensator of nuclear fuel reactivity. Incorrect
See: Boron 10
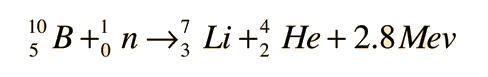
This equation describes neutron capture in the boron, which is diluted in the coolant. Boric acid is used in nuclear power plants as a long-term compensator of nuclear fuel reactivity. -
Question 18 of 20
18. Question
Which of the following charged particle reactions is very important in reactivity control?
Correct
See: Boron 10
See: Charged Particle Reactions
Explanation: Most of (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission. Therefore boron can be used as neutron absorber in case chemical shim, burnable absorbers or control rods. It can be also used as the neutron converter in neutron detectors, because its cross-section is very high and produces energetic alpha particles.
Incorrect
See: Boron 10
See: Charged Particle Reactions
Explanation: Most of (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission. Therefore boron can be used as neutron absorber in case chemical shim, burnable absorbers or control rods. It can be also used as the neutron converter in neutron detectors, because its cross-section is very high and produces energetic alpha particles.
-
Question 19 of 20
19. Question
Which of the following charged particle reactions is very important in radiation protection?
Correct
See: Charged Particle Reactions
Explanation: This nuclear reaction continually take place especially in the earth’s atmosphere, forming equilibrium amounts of the radionuclide 14C. In nuclear power plants, it is important especially from radiation protection point of view. The reaction is responsible for most of the radiation dose delivered to the human body by thermal neutrons. The nitrogen atoms are contained in proteins, therefore if the human body is exposed to thermal neutrons, then these thermal neutrons may be absorbed by 14N, causing a proton emission. Protons are directly ionizing particles and deposit their energy over a very short distance in the body tissue.
Incorrect
See: Charged Particle Reactions
Explanation: This nuclear reaction continually take place especially in the earth’s atmosphere, forming equilibrium amounts of the radionuclide 14C. In nuclear power plants, it is important especially from radiation protection point of view. The reaction is responsible for most of the radiation dose delivered to the human body by thermal neutrons. The nitrogen atoms are contained in proteins, therefore if the human body is exposed to thermal neutrons, then these thermal neutrons may be absorbed by 14N, causing a proton emission. Protons are directly ionizing particles and deposit their energy over a very short distance in the body tissue.
-
Question 20 of 20
20. Question
Q-value of the reaction is:
Correct
See: Q-value
The Q-value of the reaction is given by:
Q = [ma + mA – (mb + mB)]c2
which is the same as the excess kinetic energy of the final products:
Q = Tfinal – Tinitial
= Tb + TB – (Ta + TA)
For reactions in which there is an increase in the kinetic energy of the products Q is positive. The positive Q reactions are said to be exothermic (or exergic). There is a net release of energy, since the kinetic energy of the final state is greater than the kinetic energy of the initial state.
For reactions in which there is a decrease in the kinetic energy of the products Q is negative. The negative Q reactions are said to be endothermic (or endoergic) and they require a net energy input.
Incorrect
See: Q-value
The Q-value of the reaction is given by:
Q = [ma + mA – (mb + mB)]c2
which is the same as the excess kinetic energy of the final products:
Q = Tfinal – Tinitial
= Tb + TB – (Ta + TA)
For reactions in which there is an increase in the kinetic energy of the products Q is positive. The positive Q reactions are said to be exothermic (or exergic). There is a net release of energy, since the kinetic energy of the final state is greater than the kinetic energy of the initial state.
For reactions in which there is a decrease in the kinetic energy of the products Q is negative. The negative Q reactions are said to be endothermic (or endoergic) and they require a net energy input.