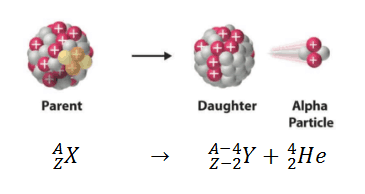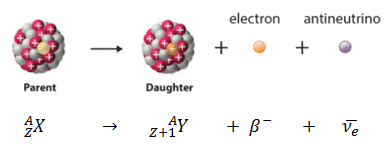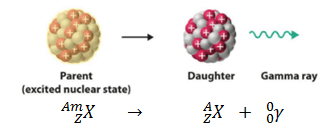In nuclear physics and physics of nuclear decays, the disintegrating nucleus is usually referred to as the parent nucleus and the nucleus remaining after the event as the daughter nucleus.
Parent Nucleus – Daughter Nucleus in Alpha Decay
For example, alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom. This transition can be characterized as:
As can be seen, the daughter nucleus’s atomic number (as well the mass number) is lower than the atomic number of the parent nucleus.
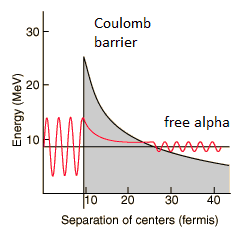 By 1928, George Gamow (and independently by Ronald Gurney and Edward Condon) had solved the theory of alpha decay via quantum tunneling. They assumed that the alpha particle and the daughter nucleus exist within the parent nucleus prior to its dissociation, namely the decay of quasistationary states (QS). A quasistationary state is defined as a long-lived state that eventually decays. Initially, the alpha cluster oscillates in the potential of the daughter nucleus, with the Coulomb potential preventing their separation. The alpha particle is trapped in a potential well by the nucleus. Classically, it is forbidden to escape, but according to the (then) newly discovered principles of quantum mechanics, it has a tiny (but non-zero) probability of “tunneling” through the barrier and appearing on the other side to escape the nucleus. Using the tunneling mechanism, Gamow, Condon, and Gurney calculated the penetrability of the tunneling α particle through the Coulomb barrier, finding the lifetimes of some α emitting nuclei.
By 1928, George Gamow (and independently by Ronald Gurney and Edward Condon) had solved the theory of alpha decay via quantum tunneling. They assumed that the alpha particle and the daughter nucleus exist within the parent nucleus prior to its dissociation, namely the decay of quasistationary states (QS). A quasistationary state is defined as a long-lived state that eventually decays. Initially, the alpha cluster oscillates in the potential of the daughter nucleus, with the Coulomb potential preventing their separation. The alpha particle is trapped in a potential well by the nucleus. Classically, it is forbidden to escape, but according to the (then) newly discovered principles of quantum mechanics, it has a tiny (but non-zero) probability of “tunneling” through the barrier and appearing on the other side to escape the nucleus. Using the tunneling mechanism, Gamow, Condon, and Gurney calculated the penetrability of the tunneling α particle through the Coulomb barrier, finding the lifetimes of some α emitting nuclei.
Parent Nucleus – Daughter Nucleus in Beta Decay
A nucleus emits a beta particle that loses an electron (or positron). In this case, the mass number of the daughter nucleus remains the same, but the daughter nucleus will form a different element. This transition can be characterized as:
Parent Nucleus – Daughter Nucleus in Gamma Decay
In nuclear physics and chemistry, the various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Nuclides are also characterized by their nuclear energy states (e.g., metastable nuclide 242mAm). Nuclides with equal proton number and equal mass number (thus making them by definition the same isotope) but different energy states are known as isomers. We usually indicate isomers with a superscript m. As a result, in gamma decay, parent and daughter nuclei have equal proton numbers and equal mass numbers but differ in their energy states. This transition can be characterized as: