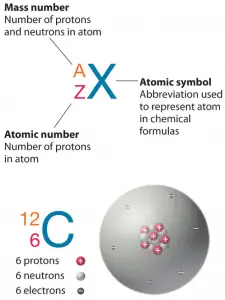
In nuclear physics and nuclear chemistry, the various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Nuclides are also characterized by their nuclear energy states (e.g., metastable nuclide 242mAm). Each nuclide is denoted by the chemical symbol of the element (this specifies Z) with the atomic mass number as a superscript. Hydrogen (H), for example, consists of one electron and one proton. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. The total number of nucleons, protons, and neutrons in a nucleus are equal to Z + N = A, where A is called the atomic mass number.
 Isobars are nuclides of different elements with the same mass number (same number of nucleons). An example of a series of isobars would be Te-135, I-135, and Xe-135, responsible for xenon poisoning in nuclear reactors. The nuclei of these nuclides all contain 135 nucleons; however, they contain varying numbers of protons and neutrons.
Isobars are nuclides of different elements with the same mass number (same number of nucleons). An example of a series of isobars would be Te-135, I-135, and Xe-135, responsible for xenon poisoning in nuclear reactors. The nuclei of these nuclides all contain 135 nucleons; however, they contain varying numbers of protons and neutrons.
The term “isobars” (originally “isobares”) for nuclides was suggested by British chemist Alfred Walter Stewart in 1918. It is derived from the Greek word isos, meaning “equal,” and baros, meaning “weight.”
As can be seen, isobars have the same mass number. The atomic mass number determines especially the atomic mass of atoms. The mass number is different for each different isotope of a chemical element. The mass number is written either after the element name or as a superscript to the left of an element’s symbol. For example, the most common isotope of carbon is carbon-12 or 12C.
For 12C, the atomic mass is exactly 12u since the atomic mass unit is defined from it. The isotopic mass usually differs for other isotopes and is usually within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63, and an isotopic mass in its nuclear ground state is 62.91367 u.
There are two reasons for the difference between mass number and isotopic mass, known as the mass defect:
- The neutron is slightly heavier than the proton, increasing the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons.
- The nuclear binding energy varies between nuclei, and a nucleus with greater binding energy has lower total energy and a lower mass according to Einstein’s mass-energy equivalence relation E = mc2. For 63Cu, the atomic mass is less than 63, so this must be the dominant factor.
l