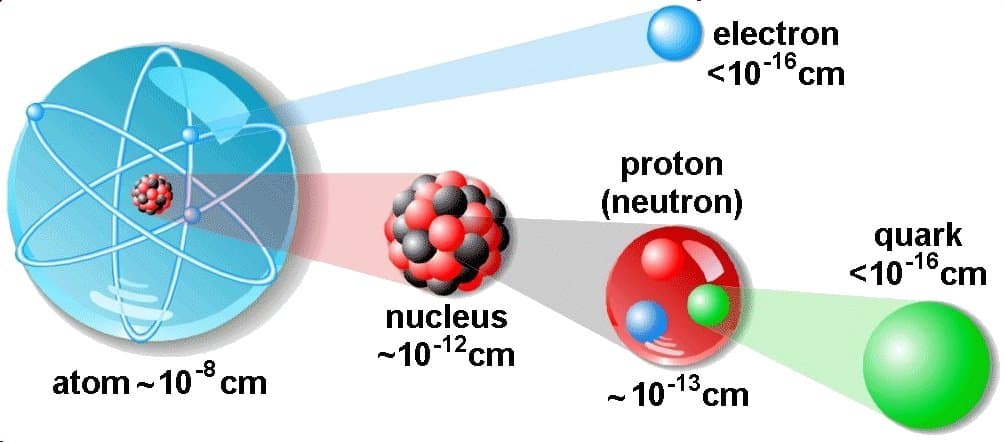
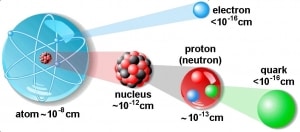
The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The total number of protons in the nucleus is called the atom’s atomic number. It is given the symbol Z. Therefore, the total electrical charge of the nucleus is +Ze, where e (elementary charge) equals 1,602 x 10-19 coulombs. In a neutral atom, there are as many electrons as protons moving about the nucleus. It is the electrons responsible for the chemical behavior of atoms and which identify the various chemical elements.
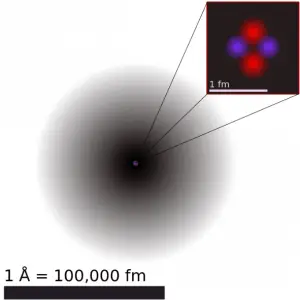
Source: chemwiki.ucdavis.edu
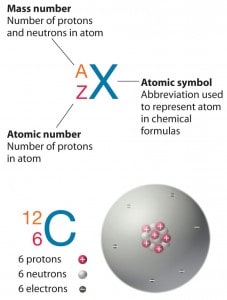
Hydrogen (H), for example, consists of one electron and one proton. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. The total number of nucleons, protons, and neutrons in a nucleus are equal to Z + N = A, where A is called the atomic mass number. The various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Each nuclide is denoted by the element’s chemical symbol (this specifies Z) with the atomic mass number as superscript.
Thus the symbol 1H refers to the nuclide of hydrogen with a single proton as a nucleus. 2H is the hydrogen nuclide with a neutron and a proton in the nucleus (2H is also called deuterium or heavy hydrogen). Atoms such as 1H, 2H whose nuclei contain the same number of protons but the different number of neutrons (different A), are known as isotopes. Uranium, for instance, has three isotopes occurring in nature – 238U, 235U, and234U. The stable isotopes (plus a few of the unstable isotopes) are the atoms found in the naturally occurring elements in nature. However, they are not found in equal amounts. Some isotopes of a given element are more abundant than others. For example, 99,27% of naturally occurring uranium atoms are the isotope 238U, 0,72% are the isotope 235U, and 0,0055% are the isotope 234U. The exact structure of atoms is described by Atomic Theory and the Theory of Nuclear Structure.
- Atomic Theory. Atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. The word atom comes from the Ancient Greek adjective atomos, meaning “uncuttable”. Today it is known that also atoms are divisible. Atomic Theory consists of many models and discoveries, which gradually formed this theory.
- Theory of Nuclear Structure. Understanding the structure of the atomic nucleus is one of the central challenges in modern nuclear physics.