 Since there are positive and negative charges (poles), these charges tend to cancel each other out. Electromagnetism includes the electrostatic force acting between charged particles at rest and the combined effect of electric and magnetic forces acting between charged particles moving relative to each other.
Since there are positive and negative charges (poles), these charges tend to cancel each other out. Electromagnetism includes the electrostatic force acting between charged particles at rest and the combined effect of electric and magnetic forces acting between charged particles moving relative to each other.
The photon, the quantum of electromagnetic radiation, is an elementary particle that is the force carrier of the electromagnetic force. Photons are gauge bosons with no electric charge or rest mass and one spin unit. Common to all photons is the speed of light, the universal constant of physics. In empty space, the photon moves at c (the speed of light – 299 792 458 meters per second).
Forces between static electrically charged particles are governed by Coulomb’s law. Coulomb’s Law can be used to calculate the force between charged particles (e.g., two protons). The electrostatic force is directly proportional to the electrical charges of the two particles and inversely proportional to the square of the distance between the particles. Coulomb’s Law is stated as the following equation.
Both Coulomb’s law and the magnetic force are summarized in the Lorentz force law. Fundamentally, both magnetic and electric forces are manifestations of an exchange force involving the exchange of photons.
The electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life. The chemical properties of atoms and molecules are determined by the number of protons, in fact, by the number and arrangement of electrons. The configuration of these electrons follows the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. In fact, the forces of electric attraction and repulsion of electric charges are so dominant over the other three fundamental forces that they can be considered negligible as determiners of atomic and molecular structure.
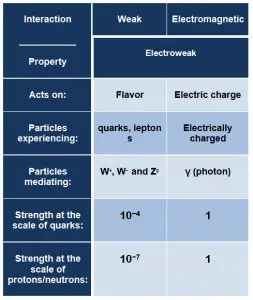
Although electromagnetic force is considered one of the four fundamental forces, at high energy, the weak force, and electromagnetic force are unified as a single electroweak force. The unification energy is on the order of 100 GeV. At low energies, electromagnetic and weak interaction appear to be very different. The electroweak theory is very important for modern cosmology. In the history of the universe, during the quark epoch (shortly after the Big Bang), the unified force broke into the two separate forces as the universe cooled.
For contributions to unifying the weak and electromagnetic interaction between elementary particles, Abdus Salam, Sheldon Glashow, and Steven Weinberg were awarded the 1979 Nobel Prize in Physics.
Example: Electromagnetic Force
Noteworthy, in four liters of water, there is about 2.1 x 108C of total electron charge. Thus, if we place two bottles a meter apart, the electrons in one of the bottles repel those in the other bottle with a force of 4.1 x 1026N. This tremendous force is comparable to the force that the planet Earth would weigh if weighed on another Earth. Ut, as was written, there are also positive (protons), and these charges tend to cancel each other out.
