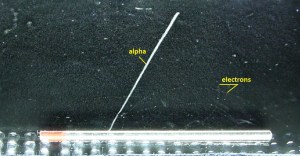
Alpha particles are energetic nuclei of helium. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths. In nuclear reactors, they are produced in the fuel (alpha decay of heavy nuclei). Alpha particles are commonly emitted by all of the heavy radioactive nuclei occurring in nature (uranium, thorium, or radium), as well as the transuranic elements (neptunium, plutonium, or americium). Especially energetic alpha particles (except artificially accelerated helium nuclei) are produced in a nuclear process known as ternary fission. In this process, the uranium nucleus is split into three charged particles (fission fragments) instead of the normal two. The smallest fission fragments most probably (90% probability) is an extra energetic alpha particle.
Characteristics of Alpha Particles
Key characteristics of alpha particles are summarized in few following points:
- Alpha particles are energetic nuclei of helium, and they are relatively heavy and carry a double positive charge.
- Typical alpha particles have a kinetic energy of about 5 MeV. This is due to the nature of alpha decay.
- Pure alpha decay is very rare. Alpha decay is frequently accompanied by gamma radiation.
- Alpha particles interact with matter primarily through coulomb forces (ionization and excitation of matter) between their electrons’ positive and negative charge of the electrons from atomic orbitals.
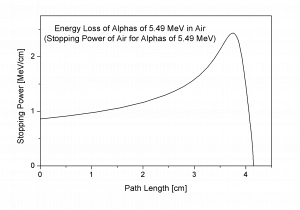
- Alpha particles heavily ionize matter, and they quickly lose their kinetic energy. Therefore alpha particles have very short ranges. On the other hand, they deposit all their energies along their short paths.
- For example, the ranges of a 5 MeV alpha particle (most have such initial energy) are approximately 0,002 cm in aluminium alloy or approximately 3.5 cm in air.
- The Bethe formula well describes the stopping power.
- The Bragg curve is typical for alpha particles and other heavy-charged particles and describes the energy loss of ionizing radiation during travel through matter.