Source: wikipedia.org
The neutrons exist in the nuclei of typical atoms, along with their positively charged counterparts, the protons. Neutrons and protons, commonly called nucleons, are bound together in the atomic nucleus, where they account for 99.9 percent of the atom’s mass. Research in high-energy particle physics in the 20th century revealed that neither the neutron nor the proton is not the smallest building block of matter. Protons and neutrons also have their structure. Inside the protons and neutrons, we find true elementary particles called quarks. Within the nucleus, protons and neutrons are bound together through a strong force. This fundamental interaction governs the behavior of the quarks that make up the individual protons and neutrons.
The competition between two fundamental interactions determines nuclear stability. Protons and neutrons are attracted each other via the strong force. On the other hand, protons repel each other via the electric force due to their positive charge. Therefore neutrons within the nucleus act somewhat like nuclear glue. Neutrons attract each other and protons, which helps offset the electrical repulsion between protons. There are only certain combinations of neutrons and protons, which form stable nuclei. For example, the most common nuclide of the common chemical element lead (Pb) has 82 protons and 126 neutrons.
Source: hyperphysics.phy-astr.gsu.edu
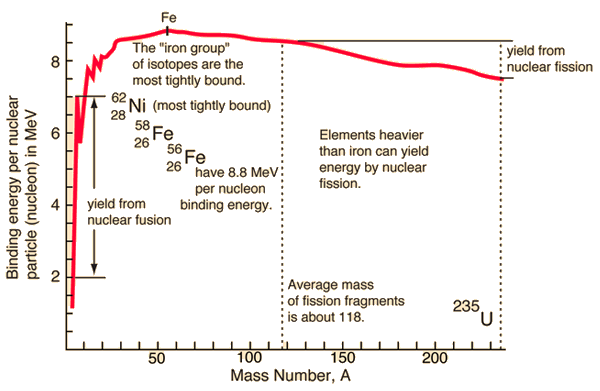
Because of the strength of the nuclear force at short distances, the nuclear binding energy (the energy required to disassemble a nucleus of an atom into its component parts) of nucleons is more than seven orders of magnitude larger than the electromagnetic energy binding electrons in atoms. Therefore, nuclear reactions (such as nuclear fission or nuclear fusion) have an energy density of more than 10 000 000x that of chemical reactions.
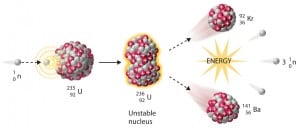
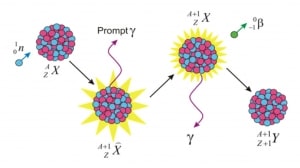
Knowledge of the behavior and properties of neutrons is essential to the production of nuclear power. The neutron was discovered in 1932. The fact that neutrons might act to form a nuclear chain reaction was realized quickly after that. When nuclear fission was discovered in 1938, it became clear that if a fission reaction produced free neutrons, each of these neutrons might cause further fission reaction in a cascade known as a chain reaction. Knowledge of cross-sections (the key parameter representing the probability of interaction between a neutron and a nucleus) became crucial for designing reactor cores and the first nuclear weapon (Trinity, 1945).
Structure of the Neutron

Neutrons and protons are classified as hadrons, subatomic particles subject to the strong force, and baryons since they are composed of three quarks. The neutron is a composite particle made of two down quarks with charge −⅓ e and one up quark with charge +⅔ e. Since the neutron has no net electric charge, it is not affected by electric forces, but the neutron does have a slight distribution of electric charge within it. This results in a non-zero magnetic moment (dipole moment) of the neutron. Therefore the neutron also interacts via electromagnetic interaction but is much weaker than the proton.
The mass of the neutron is 939.565 MeV/c2, whereas the mass of the three quarks is only about 12 MeV/c2 (only about 1% of the mass-energy of the neutron). Like the proton, most of the mass (energy) of the neutron is in the form of the strong nuclear force energy (gluons). The quarks of the neutron are held together by gluons, the exchange particles for the strong nuclear force. Gluons carry the color charge of the strong nuclear force.
See also: Structure of the Neutron
Properties of the Neutron
Key properties of neutrons are summarized below:
- Mean square radius of a neutron is ~ 0.8 x 10-15m (0.8 fermi)
- The mass of the neutron is 939.565 MeV/c2
- Neutrons are ½ spin particles – fermionic statistics
- Neutrons are neutral particles – no net electric charge.
- Neutrons have a non-zero magnetic moment.
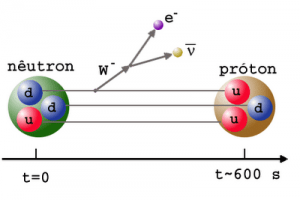
- Free neutrons (outside a nucleus) are unstable and decay via beta decay. The decay of the neutron involves the weak interaction and is associated with a quark transformation (a down quark is converted to an up quark).
- The mean lifetime of a free neutron is 882 seconds (i.e., the half-life is 611 seconds ).
- A natural neutron background of free neutrons exists everywhere on Earth. It is caused by muons produced in the atmosphere, where high-energy cosmic rays collide with particles of Earth’s atmosphere.
- Neutrons cannot directly cause ionization. Neutrons ionize matter only indirectly.
- Neutrons can travel hundreds of feet in the air without any interaction. Neutron radiation is highly penetrating.
- Neutrons trigger nuclear fission.
- The fission process produces free neutrons (2 or 3).
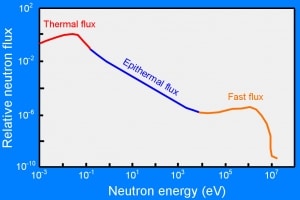
- Thermal or cold neutrons have wavelengths similar to atomic spacings. They can be used in neutron diffraction experiments to determine the atomic and/or magnetic structure of a material.
See also: Properties of the Neutron.
Interactions of Neutrons with Matter
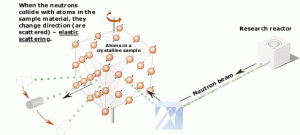
 Neutrons are neutral particles. Therefore they travel in straight lines, deviating from their path only when they collide with a nucleus to be scattered into a new direction or absorbed. Neither the electrons surrounding (atomic electron cloud) a nucleus nor the electric field caused by a positively charged nucleus affect a neutron’s flight. In short, neutrons collide with nuclei, not with atoms. A very descriptive feature of the transmission of neutrons through bulk matter is the mean free path length (λ – lambda), which is the mean distance a neutron travels between interactions. It can be calculated from the following equation:
Neutrons are neutral particles. Therefore they travel in straight lines, deviating from their path only when they collide with a nucleus to be scattered into a new direction or absorbed. Neither the electrons surrounding (atomic electron cloud) a nucleus nor the electric field caused by a positively charged nucleus affect a neutron’s flight. In short, neutrons collide with nuclei, not with atoms. A very descriptive feature of the transmission of neutrons through bulk matter is the mean free path length (λ – lambda), which is the mean distance a neutron travels between interactions. It can be calculated from the following equation:
λ=1/Σ
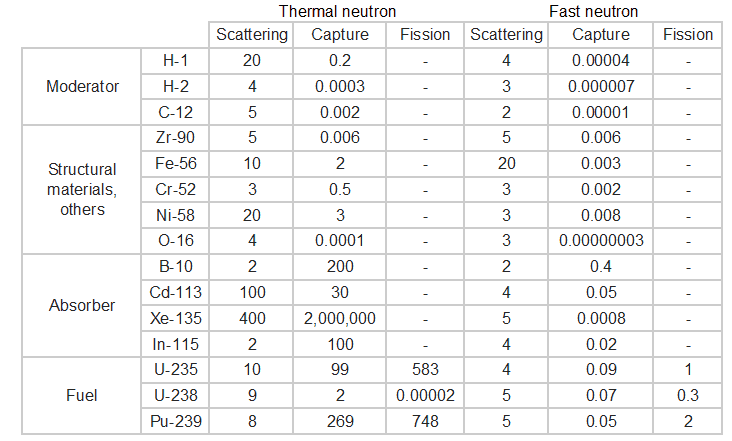
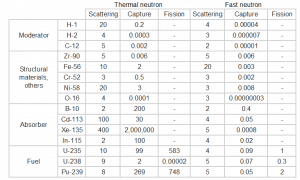
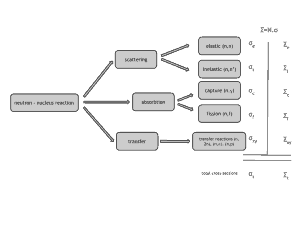
Neutrons may interact with nuclei in one of following ways:
Types of neutron-nuclear reactions
Detection of Neutrons
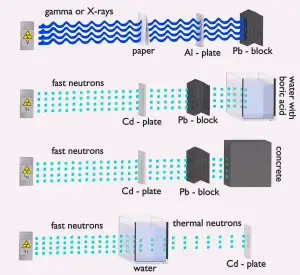
Since the neutrons are electrically neutral particles, they are mainly subject to strong nuclear forces but not electric forces. Therefore neutrons are not directly ionizing, and they usually have to be converted into charged particles before they can be detected. Generally, every type of neutron detector must be equipped with a converter (to convert neutron radiation to common detectable radiation) and one of the conventional radiation detectors (scintillation detector, gaseous detector, semiconductor detector, etc.).
Neutron converters
Two basic types of neutron interactions with matter are for this purpose available:
- Elastic scattering. The free neutron can be scattered by a nucleus, transferring some of its kinetic energy to the nucleus. If the neutron has enough energy to scatter off nuclei, the recoiling nucleus ionizes the material surrounding the converter. Only hydrogen and helium nuclei are light enough for practical application. The conventional detector can collect charges produced in this way to produce a detected signal. Neutrons can transfer more energy to light nuclei. This method is appropriate for detecting fast neutrons (fast neutrons do not have a high cross-section for absorption), allowing the detection of fast neutrons without a moderator.
- Neutron absorption. This is a common method allowing the detection of neutrons of the entire energy spectrum. This method is based on various absorption reactions (radiative capture, nuclear fission, rearrangement reactions, etc.). The neutron is absorbed by target material (converter), emitting secondary particles such as protons, alpha particles, beta particles, photons (gamma rays), or fission fragments. Some reactions are threshold reactions (requiring minimum energy of neutrons), but most reactions occur at epithermal and thermal energies. That means the moderation of fast neutrons is required leading to poor energy information of the neutrons. The most common nuclei for the neutron converter material are:
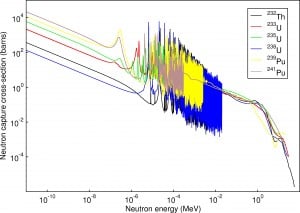
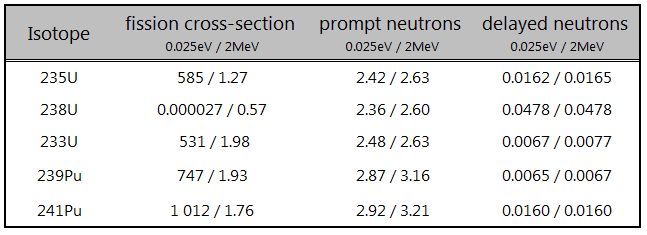
- 10B(n,α). Where the neutron capture cross-section for thermal neutrons is σ = 3820 barns, and the natural boron has an abundance of 10B 19,8%.
- 3He(n,p). Where the neutron capture cross-section for thermal neutrons is σ = 5350 barns, and the natural helium has an abundance of 3He 0.014%.
- 6Li(n,α). Where the neutron capture cross-section for thermal neutrons is σ = 925 barns, and the natural lithium has an abundance of 6Li 7,4%.
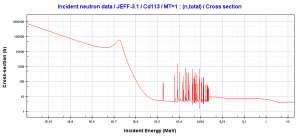
- 113Cd(n,ɣ). Where the neutron capture cross-section for thermal neutrons is σ = 20820 barns, and the natural cadmium has abundance of 113Cd 12,2%.
- 235U(n,fission). Where the fission cross-section for thermal neutrons is σ = 585 barns, and the natural uranium has an abundance of 235U 0.711%. Uranium as a converter produces fission fragments which are heavy charged particles. This has a significant advantage. The heavy charged particles (fission fragments) create a high output signal because the fragments deposit a large amount of energy in a detector-sensitive volume. This allows easy discrimination of the background radiation (e.i. gamma radiation). This important feature can be used, for example, in a nuclear reactor power measurement, where a significant gamma background accompanies the neutron field.
See also: Detection of Neutrons
Neutron Sources
A neutron source is any device that emits neutrons. Neutron sources have many applications, and they can be used in research, engineering, medicine, petroleum exploration, biology, chemistry, and nuclear power. A number of factors characterize a neutron source:
- Significance of the source
- Intensity. The rate of neutrons emitted by the source.
- Energy distribution of emitted neutrons.
- Angular distribution of emitted neutrons.
- Mode of emission. Continuous or pulsed operation.
Classification by significance of the source
- Large (Significant) neutron sources
- Nuclear Reactors. There are nuclei that can undergo fission on their own spontaneously, but only certain nuclei, like uranium-235, uranium-233, and plutonium-239, can sustain a fission chain reaction. This is because these nuclei release neutrons when they break apart, which can induce the fission of other nuclei. Uranium-235, which exists as 0.7% of naturally occurring uranium, undergoes nuclear fission with thermal neutrons with the production of, on average, 2.4 fast neutrons and the release of ~ 180 MeV of energy per fission. Free neutrons released by each fission play a very important role as a trigger of the reaction, but they can also be used for another purpose. For example, one neutron is required to trigger further fission. Part of free neutrons (let say 0.5 neutrons/fission) is absorbed in other material, but an excess of neutrons (0.9 neutrons/fission) can leave the surface of the reactor core and can be used as a neutron source.
-
- Fusion Systems. Nuclear fusion is a nuclear reaction in which two or more atomic nuclei (e.g.,, D+T) collide at very high energy and fuse together. Thy byproduct of DT fusion is a free neutron (see picture). Therefore, nuclear fusion reactions can produce large quantities of neutrons.
- Spallation Sources. A spallation source is a high-flux neutron source in which protons that have been accelerated to high energies hit a heavy target material, causing the emission of neutrons. The reaction occurs above a certain energy threshold for the incident particle, which is typically 5 – 15 MeV.
- Medium neutron sources
- Bremssstrahlung from Electron Accelerators / Photofission. When slowed down rapidly in a heavy target, energetic electrons emit intense gamma radiation during the deceleration process. This is known as Bremsstrahlung or braking radiation. The interaction of the gamma radiation with the target produces neutrons via the (γ,n) reaction, or the (γ, fission) reaction when a fissile target is used. e-→Pb → γ→ Pb →(γ,n) and (γ,fission). The Bremsstrahlung γ energy exceeds the binding energy of the “last” neutron in the target. A source strength of 1013 neutrons/second produced in short (i.e., < 5 μs) pulses can be readily realized.
- Dense plasma focus. The dense plasma focus (DPF) is a device known as an efficient source of neutrons from fusion reactions. The dense plasma focus (DPF) mechanism is based on nuclear fusion of short-lived plasma of deuterium and/or tritium. This device produces a short-lived plasma by electromagnetic compression and acceleration that is called a pinch. This plasma is during the pinch hot and dense enough to cause nuclear fusion and the emission of neutrons.
- Light ion accelerators. Neutrons can also be produced by particle accelerators using targets of deuterium, tritium, lithium, beryllium, and other low-Z materials. In this case, the target must be bombarded with accelerated hydrogen (H), deuterium (D), or tritium (T) nuclei.
- Small neutron sources
- Neutron Generators. Neutrons are produced in the fusion of deuterium and tritium in the following exothermic reaction. 2D + 3T → 4He + n + 17.6 MeV. The neutron is produced with a kinetic energy of 14.1 MeV. This can be achieved on a small scale in the laboratory with a modest 100 kV accelerator for deuterium atoms bombarding a tritium target. Continuous neutron sources of ~1011 neutrons/second can be achieved relatively simply.
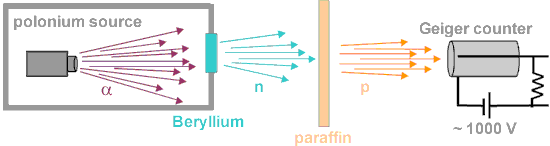
- Radioisotope source – (α,n) reactions. In certain light isotopes, the ‘last’ neutron in the nucleus is weakly bound and is released when the compound nucleus formed following α-particle bombardment decays. The bombardment of beryllium by α-particles leads to the production of neutrons by the following exothermic reaction: 4He + 9Be→12C + n + 5.7 MeV. This reaction yields a weak source of neutrons with an energy spectrum resembling that from a fission source and is used nowadays in portable neutron sources. Radium, plutonium, or americium can be used as an α-emitter.
- Radioisotope source – (γ,n) reactions. (γ,n) reactions can also be used for the same purpose. In this type of source, because of the greater range of the γ-ray, the two physical components of the source can be separated, making it possible to ‘switch off’ the reaction if so required by removing the radioactive source from the beryllium. (γ,n) sources produce monoenergetic neutrons, unlike (α,n) sources. The (γ,n) source uses antimony-124 as the gamma emitter in the following endothermic reaction.
124Sb→124Te + β− + γ
γ + 9Be→8Be + n – 1.66 MeV
-
- Radioisotope source – spontaneous fission. Certain isotopes undergo spontaneous fission with the emission of neutrons. The most commonly used spontaneous fission source is the radioactive isotope californium-252. Cf-252 and other spontaneous fission neutron sources are produced by irradiating uranium or another transuranic element in a nuclear reactor, where neutrons are absorbed in the starting material and its subsequent reaction products, transmuting the starting material into the SF isotope.
See also: Neutron Sources
See also: Source Neutrons