Alpha radiation consists of alpha particles that are energetic nuclei of helium. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths.
Beta radiation consists of free electrons or positrons at relativistic speeds, which are termed beta particles. Beta particles (electrons) are much smaller than alpha particles. They carry a single negative charge. They are more penetrating than alpha particles, but thin aluminum metal can stop them. They can travel several meters but deposit less energy at any point along their paths than alpha particles.
Gamma rays, also known as gamma radiation, refer to electromagnetic radiation (no rest mass, no charge) of very high energies. Gamma rays are high-energy photons with very short wavelengths and thus very high frequency. Since the gamma rays are in substance only very high-energy photons, they are very penetrating matter and are thus biologically hazardous. Gamma rays can travel thousands of feet in the air and can easily pass through the human body.
Alpha Particles
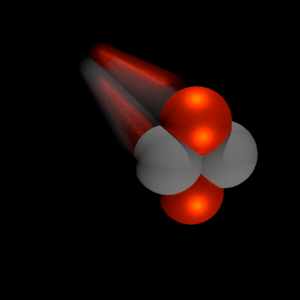 Alpha particles are energetic nuclei of helium. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths.
Alpha particles are energetic nuclei of helium. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths.
In nuclear reactors, they are produced in the fuel (alpha decay of heavy nuclei). Alpha particles are commonly emitted by all of the heavy radioactive nuclei occurring in nature (uranium, thorium, or radium), as well as the transuranic elements (neptunium, plutonium, or americium).
Especially energetic alpha particles (except artificially accelerated helium nuclei) are produced in a nuclear process known as ternary fission. In this process, the uranium nucleus is split into three charged particles (fission fragments) instead of the normal two. The smallest fission fragments most probably (90% probability) is an extra energetic alpha particle.
See also: Alpha Particle
See also: Interaction of Heavy Particles with Matter
See also: Shielding of Alpha Particles
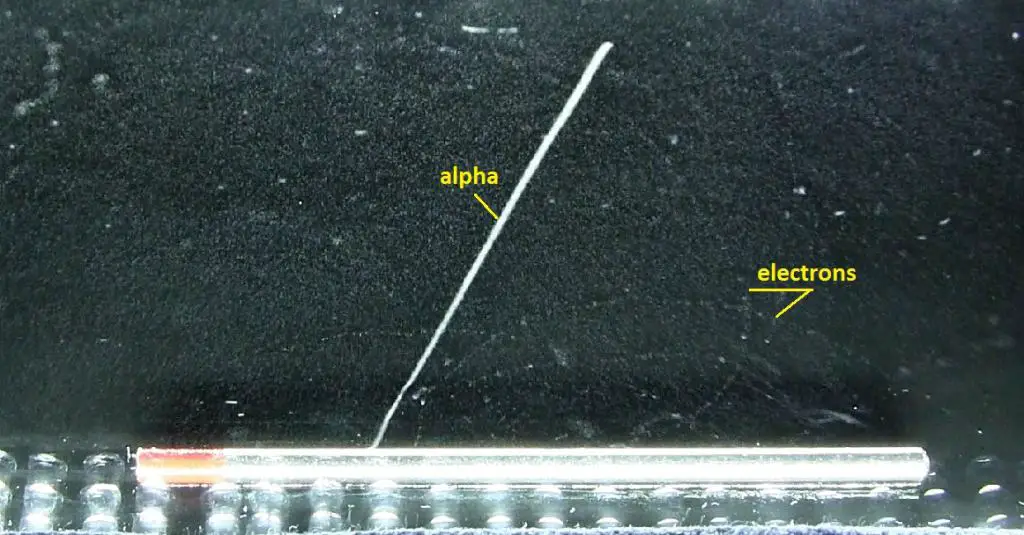
Source: wikipedia.org
Beta Particles
Description Beta Particles
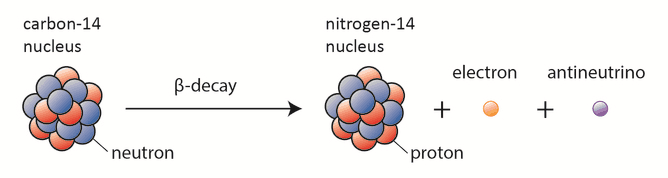
Beta particles are high-energy, high-speed electrons or positrons emitted by certain fission fragments or certain primordial radioactive nuclei such as potassium-40. The beta particles are a form of ionizing radiation, also known as beta rays. The production of beta particles is termed beta decay. There are two forms of beta decay, electron decay (β− decay) and positron decay (β+ decay). A nuclear reactor occurs especially the β− decay because the common feature of the fission products is an excess of neutrons (see Nuclear Stability). An unstable fission fragment with the excess of neutrons undergoes β− decay, where the neutron is converted into a proton, an electron, and an electron antineutrino.
See also: Beta Particle
See also: Interaction of Beta Particles with Matter
See also: Shielding of Beta Radiation
Gamma Rays
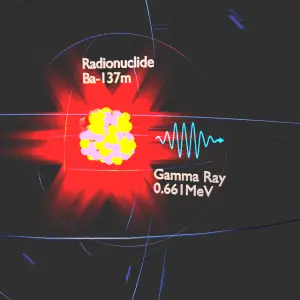
Gamma rays, also known as gamma radiation, refer to electromagnetic radiation (no rest mass, no charge) of very high energies. Gamma rays are high-energy photons with very short wavelengths and thus very high frequency. Since the gamma rays are in substance only very high-energy photons, they are very penetrating matter and are thus biologically hazardous. Gamma rays can travel thousands of feet in the air and can easily pass through the human body.
Gamma rays are emitted by unstable nuclei in their transition from a high-energy state to a lower state known as gamma decay. In most practical laboratory sources, the excited nuclear states are created in the decay of a parent radionuclide. Therefore a gamma decay typically accompanies other forms of decay, such as alpha or beta decay.
Radiation and also gamma rays are all around us. In, around, and above the world we live in. It is a part of our natural world that has been here since the birth of our planet. Natural sources of gamma rays on Earth are, among other things, gamma rays from naturally occurring radionuclides, particularly potassium-40. Potassium-40 is a radioactive isotope of potassium that has a very long half-life of 1.251×109 years (comparable to the age of Earth). This isotope can be found in soil, water also in meat and bananas. This is not the only example of a natural source of gamma rays.
See also: Gamma Rays
See also: Interaction of Gamma Radiation with Matter
See also: Shielding of Gamma Radiation