Source: wikipedia.org
Key characteristics of alpha radiation are summarized in few following points:
- Alpha particles are energetic nuclei of helium, and they are relatively heavy and carry a double positive charge.
- Typical alpha particles have a kinetic energy of about 5 MeV. This is due to the nature of alpha decay.
- Pure alpha decay is very rare, and alpha decay is frequently accompanied by gamma radiation.
- Alpha particles interact with matter primarily through coulomb forces (ionization and excitation of matter) between their electrons’ positive and negative charge of the electrons from atomic orbitals.
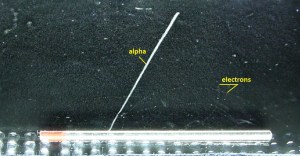
- Alpha particles heavily ionize matter, and they quickly lose their kinetic energy. Therefore alpha particles have very short ranges. On the other hand, they deposit all their energies along their short paths.
- For example, the ranges of a 5 MeV alpha particle (most have such initial energy) are approximately 0,002 cm in aluminium alloy or approximately 3.5 cm in air.
- The Bethe formula well describes the stopping power.
- The Bragg curve is typical for alpha particles and other heavy-charged particles and describes the energy loss of ionizing radiation during travel through matter.
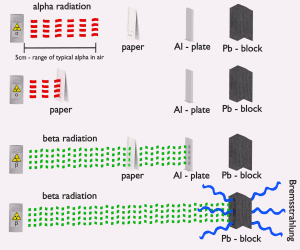
The stopping power of most materials is very high for alpha particles and heavy-charged particles. Therefore alpha particles have very short ranges. For example, the ranges of a 5 MeV alpha particle (most have such initial energy) are approximately 0,002 cm in aluminium alloy or approximately 3.5 cm in air. A thin piece of paper can stop most alpha particles. Even the dead cells in the outer layer of human skin provide adequate shielding because alpha particles can’t penetrate it.
Alpha Decay
Alpha decay (or α-decay and alpha radioactivity) represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom. Alpha decay is a quantum tunneling process. To be emitted, the alpha particle must penetrate a potential barrier. This is similar to cluster decay, in which an atomic nucleus emits a small “cluster” of neutrons and protons (e.g.,, 12C).
The height of the Coulomb barrier for nuclei of A « 200 is about 20-25 MeV. The alpha particles emitted in nuclear decay have typical energies of about 5 MeV.
Alpha Particles
 Alpha particles are energetic nuclei of helium. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths. In nuclear reactors, they are produced in the fuel (alpha decay of heavy nuclei). Alpha particles are commonly emitted by all of the heavy radioactive nuclei occurring in nature (uranium, thorium, or radium), as well as the transuranic elements (neptunium, plutonium, or americium). Especially energetic alpha particles (except artificially accelerated helium nuclei) are produced in a nuclear process known as ternary fission. In this process, the uranium nucleus is split into three charged particles (fission fragments) instead of the normal two. The smallest fission fragments most probably (90% probability) is an extra energetic alpha particle.
Alpha particles are energetic nuclei of helium. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths. In nuclear reactors, they are produced in the fuel (alpha decay of heavy nuclei). Alpha particles are commonly emitted by all of the heavy radioactive nuclei occurring in nature (uranium, thorium, or radium), as well as the transuranic elements (neptunium, plutonium, or americium). Especially energetic alpha particles (except artificially accelerated helium nuclei) are produced in a nuclear process known as ternary fission. In this process, the uranium nucleus is split into three charged particles (fission fragments) instead of the normal two. The smallest fission fragments most probably (90% probability) is an extra energetic alpha particle.
Interaction of Alpha Particles with Matter
Since the electromagnetic interaction extends over some distance, alpha particles don’t have to collide with an atom directly. They can transfer energy simply by passing close by. Alpha particles interact with matter primarily through coulomb forces between their positive charge and the negative charge of the electrons from atomic orbitals. In general, the alpha particles (like other charged particles) transfer energy mostly by:
- Excitation. The charged particle can transfer energy to the atom, raising electrons to higher energy levels.
- Ionization. Ionization can occur when the charged particle has enough energy to remove an electron. This results in the creation of ion pairs in surrounding matter.
The creation of pairs requires energy, which is lost from the kinetic energy of the alpha particle, causing it to decelerate. The positive ions and free electrons created by the passage of the alpha particle will then reunite, releasing energy in the form of heat (e.g.,, vibrational energy or rotational energy of atoms). There are considerable differences in the ways of energy loss and scattering between the passage of light-charged particles such as positrons and electrons and heavy charged particles such as fission fragments, alpha particles, muons. Most of these differences are based on the different dynamics of the collision process. In general, when a heavy particle collides with a much lighter particle (electrons in the atomic orbitals), the laws of energy and momentum conservation predict that only a small fraction of the massive particle’s energy can be transferred to the less massive particle. The actual amount of transferred energy depends on how closely the charged particles pass through the atom, and it also depends on restrictions from the quantization of energy levels.
See also: Interaction of Heavy Charged Particles with Matter
Alpha Radiation – Biological Effects
Alpha and beta particles, in general, constitute no external exposure hazard because the particles generally do not pass through the skin. On the other hand, alpha radiation is very harmful when alpha radionuclides are ingested or inhaled. Internal exposure is more dangerous than external exposure since we are carrying the source of radiation inside our bodies, and we cannot use any of the radiation protection principles (time, distance, shielding).
Studies have shown that alpha and neutron radiation cause greater biological damage for a given energy deposition per kg of tissue than gamma radiation does. It was discovered, biological effects of any radiation increase with the linear energy transfer (LET). In short, the biological damage from high-LET radiation (alpha particles, protons, or neutrons) is much greater than that from low-LET radiation (gamma rays). This is because the living tissue can more easily repair damage from radiation spread over a large area than that which is concentrated in a small area.
See also: Alpha Dosimetry
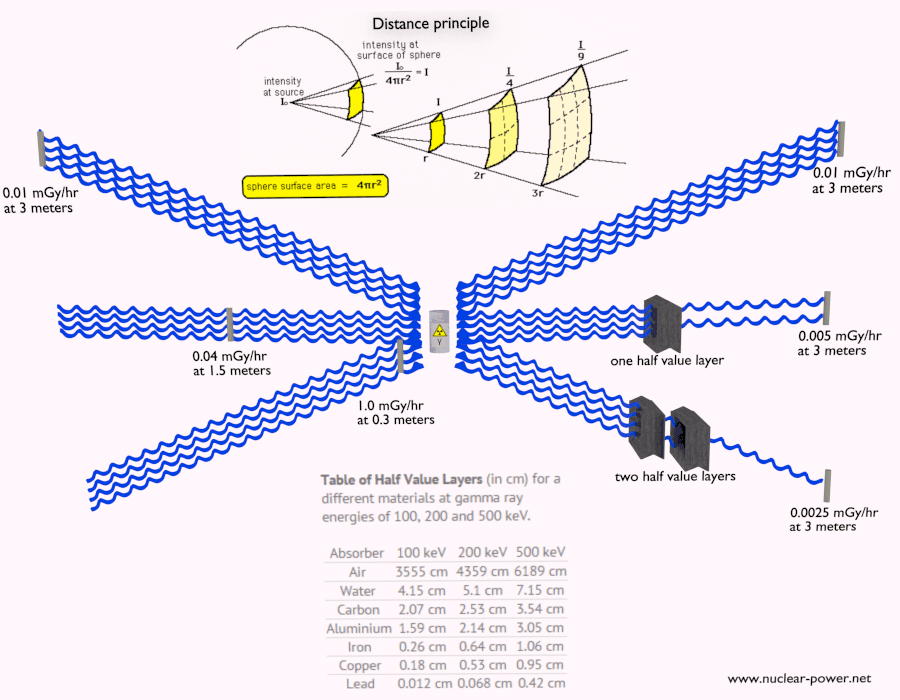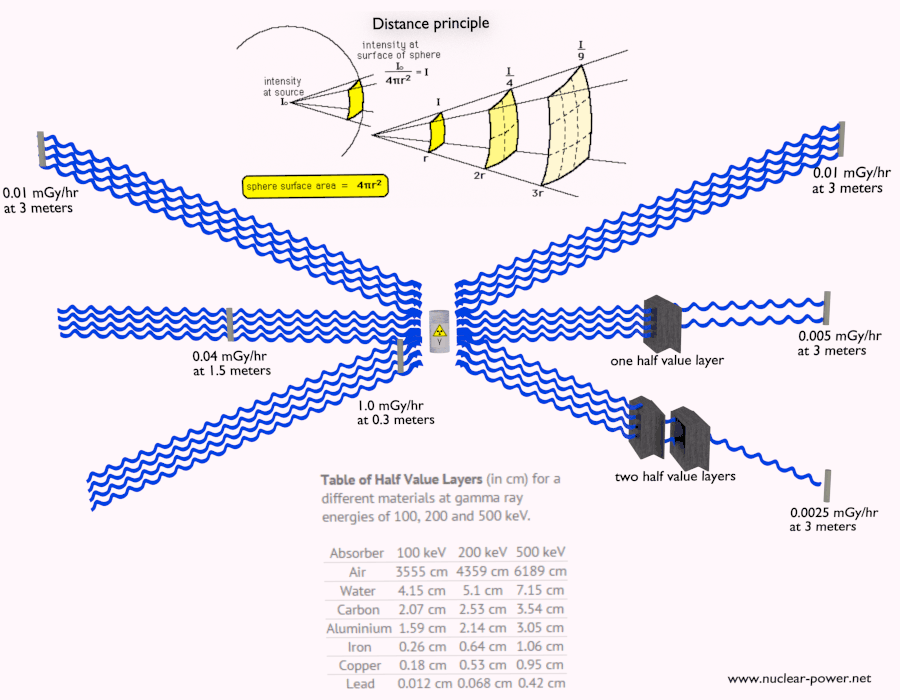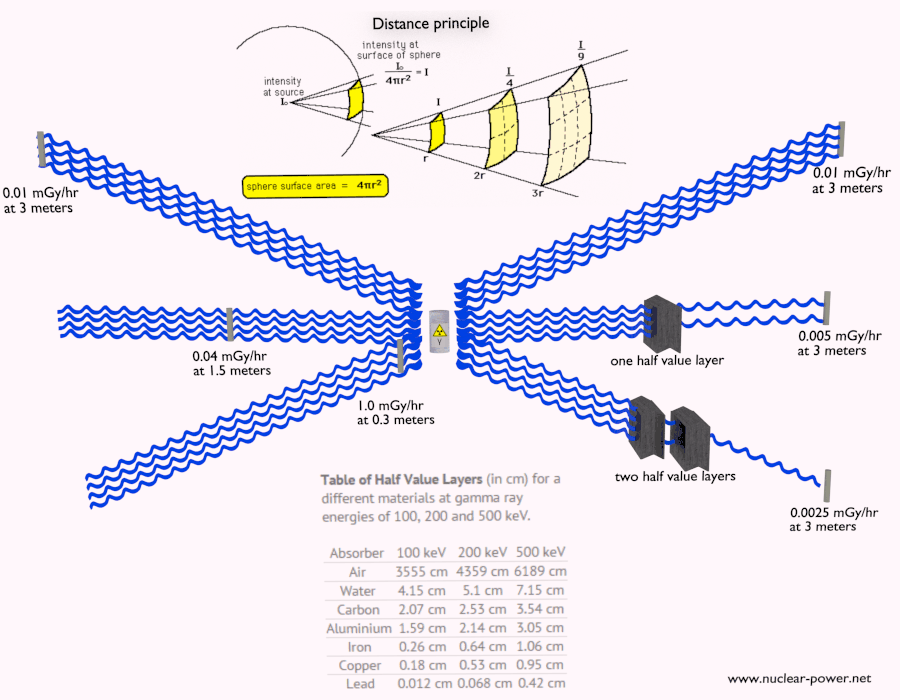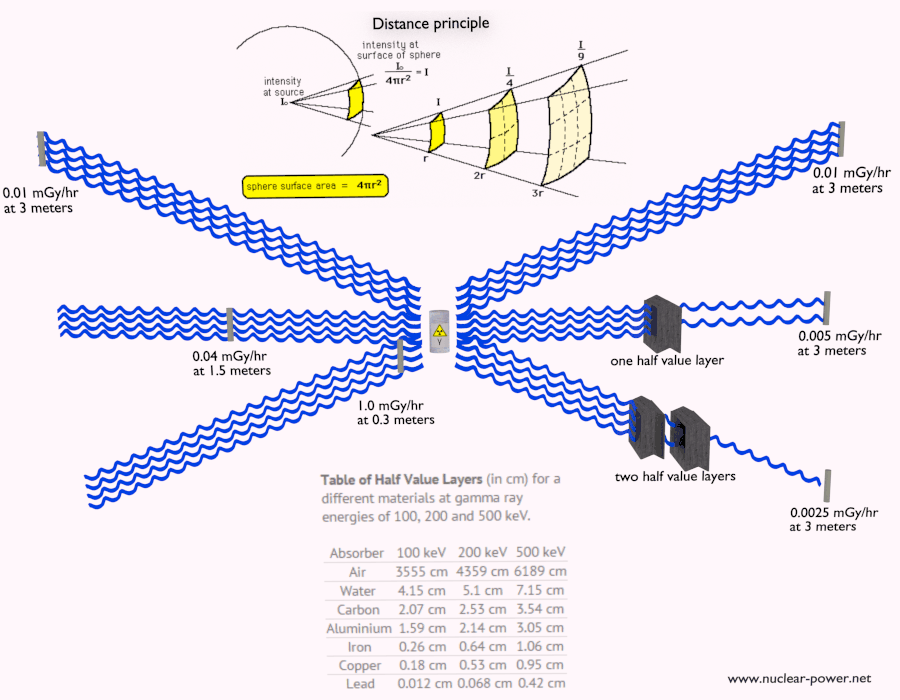
Shielding of Alpha Radiation
The shielding of alpha radiation alone does not pose a difficult problem. On the other hand, Alpha radioactive nuclides can lead to serious health hazards when ingested or inhaled (internal contamination). When they are ingested or inhaled, the alpha particles from their decay significantly harm the internal living tissue. Moreover, pure alpha radiation is very rare, and alpha decay is frequently accompanied by gamma radiation which shielding is another issue.
See also: Shielding of Alpha Radiation.