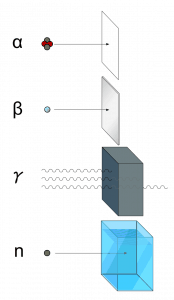
There are several forms and types of ionizing radiation. Ionizing radiation is categorized by the nature of the particles or electromagnetic waves that create the ionizing effect. These particles/waves have different ionization mechanisms and may be grouped as:
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly through fundamental interaction through the Coulomb force if they carry sufficient kinetic energy. These particles must be moving at relativistic speeds to reach the required kinetic energy. Even photons (gamma rays and X-rays) can ionize atoms directly (despite they are electrically neutral) through the Photoelectric effect and the Compton effect, but secondary (indirect) ionization is much more significant.
- Alpha radiation. Alpha radiation consists of alpha particles at high energy/speed. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths.
- Beta radiation. Beta radiation consists of free electrons or positrons at relativistic speeds. Beta particles (electrons) are much smaller than alpha particles. They carry a single negative charge. They are more penetrating than alpha particles, but thin aluminum metal can stop them. They can travel several meters but deposit less energy at any one point along their paths than alpha particles.
- Indirectly ionizing. Indirect ionizing radiation is electrically neutral particles and therefore does not interact strongly with matter. The bulk of the ionization effects are due to secondary ionizations.
- Photon radiation (Gamma or X-rays). Photon radiation consists of high-energy photons. These photons are particles/waves (Wave-Particle Duality) without rest mass or electrical charge. They can travel 10 meters or more in the air. This is long-distance compared to alpha or beta particles. However, gamma rays deposit less energy along their paths. Lead, water, and concrete stop gamma radiation. Photons (gamma rays and X-rays) can ionize atoms directly through the Photoelectric and Compton effect, where the relatively energetic electron is produced. The secondary electron will go on to produce multiple ionization events. Therefore the secondary (indirect) ionization is much more significant.
- Neutron radiation. Neutron radiation consists of free neutrons at any energies/speeds. Neutrons can be emitted by nuclear fission or by the decay of some radioactive atoms. Neutrons have zero electrical charges and cannot directly cause ionization. Neutrons ionize matter only indirectly. For example, when neutrons strike the hydrogen nuclei, proton radiation (fast protons) results. Neutrons can range from high speed, high energy particles to low speed, low energy particles (called thermal neutrons). Neutrons can travel hundreds of feet in the air without any interaction.
Sources of Radiation
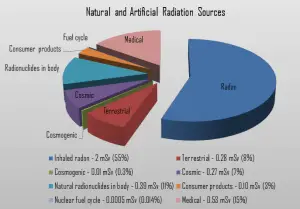 Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. From the beginning of time, all living creatures have been, and are still being, exposed to ionizing radiation. Ionizing radiation is generated through nuclear reactions, nuclear decay, very high temperature, or the acceleration of charged particles in electromagnetic fields. But in general, there are two broad categories of radiation sources:
Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. From the beginning of time, all living creatures have been, and are still being, exposed to ionizing radiation. Ionizing radiation is generated through nuclear reactions, nuclear decay, very high temperature, or the acceleration of charged particles in electromagnetic fields. But in general, there are two broad categories of radiation sources:
- Natural Background Radiation. Natural background radiation includes radiation produced by the Sun, lightning, primordial radioisotopes or supernova explosions, etc.
- Man-Made Sources of Radiation. Man-made sources include medical uses of radiation, residues from nuclear tests, industrial uses of radiation, etc.
Special Reference: Sources and effects of ionizing radiation, Annex B. UNSCEAR. New York, 2010. ISBN: 978-92-1-142274-0.