The following features of alpha particles are crucial in their shielding.
- Alpha particles are energetic nuclei of helium, and they are relatively heavy and carry a double positive charge.
- Alpha particles interact with matter primarily through coulomb forces (ionization and excitation of matter) between their electrons’ positive and negative charge of the electrons from atomic orbitals.
- Alpha particles heavily ionize matter, and they quickly lose their kinetic energy. On the other hand, they deposit all their energies along their short paths.
- The Bethe formula well describes the stopping power.
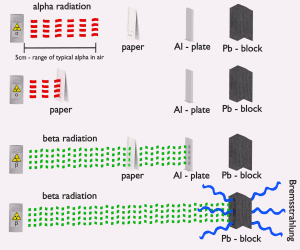 The stopping power of most materials is very high for alpha particles and heavy-charged particles. Therefore alpha particles have very short ranges. For example, the ranges of a 5 MeV alpha particle (most have such initial energy) are approximately 0,002 cm in aluminium alloy or approximately 3.5 cm in air. A thin piece of paper can stop most alpha particles. Even the dead cells in the outer layer of human skin provide adequate shielding because alpha particles can’t penetrate it.
The stopping power of most materials is very high for alpha particles and heavy-charged particles. Therefore alpha particles have very short ranges. For example, the ranges of a 5 MeV alpha particle (most have such initial energy) are approximately 0,002 cm in aluminium alloy or approximately 3.5 cm in air. A thin piece of paper can stop most alpha particles. Even the dead cells in the outer layer of human skin provide adequate shielding because alpha particles can’t penetrate it.
Therefore the shielding of alpha radiation alone does not pose a difficult problem. On the other hand, alpha radioactive nuclides can lead to serious health hazards when ingested or inhaled (internal contamination). When they are ingested or inhaled, the alpha particles from their decay significantly harm the internal living tissue. Moreover, pure alpha radiation is very rare. Alpha decay is frequently accompanied by gamma radiation which shielding is another issue.
See also: Interaction of Heavy Charged Particles with Matter.