X-rays, also known as X-radiation, refer to electromagnetic radiation (no rest mass, no charge) of high energies. X-rays are high-energy photons with short wavelengths and thus very high frequency. The radiation frequency is the key parameter of all photons because it determines the energy of a photon. Photons are categorized according to their energies, from low-energy radio waves and infrared radiation, through visible light, to high-energy X-rays and gamma rays.
Most X-rays have a wavelength ranging from 0.01 to 10 nanometers (3×1016 Hz to 3×1019 Hz), corresponding to energies in the range of 100 eV to 100 keV. X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays.
X-Ray Spectrum – Characteristic and Continuous
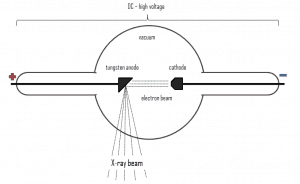 For X-rays generated by an X-ray tube, the part of the energy that is transformed into radiation varies from zero up to the maximum energy of the electron when it hits the anode. The maximum energy of the produced X-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so a 100 kV tube cannot create X-rays with an energy greater than 100 keV. When the electrons hit the target, X-rays are created by two different atomic processes:
For X-rays generated by an X-ray tube, the part of the energy that is transformed into radiation varies from zero up to the maximum energy of the electron when it hits the anode. The maximum energy of the produced X-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so a 100 kV tube cannot create X-rays with an energy greater than 100 keV. When the electrons hit the target, X-rays are created by two different atomic processes:
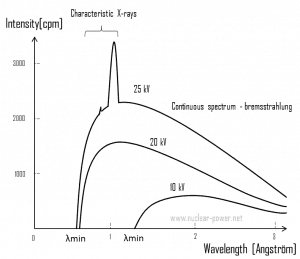 Bremsstrahlung. The bremsstrahlung is electromagnetic radiation produced by the acceleration or deceleration of an electron when deflected by strong electromagnetic fields of target high-Z (proton number) nuclei. The name bremsstrahlung comes from German, and the literal translation is ‘braking radiation.’ From classical theory, when a charged particle is accelerated or decelerated, it must radiate energy. The bremsstrahlung is one of the possible interactions of light-charged particles with matter (especially with high atomic numbers). These X-rays have a continuous spectrum. The intensity of the X-rays increases linearly with decreasing frequency, from zero at the energy of the incident electrons to the voltage on the X-ray tube. Changing the material from which the target in the tube is made does not affect the spectrum of this continuous radiation. If we were to switch from a molybdenum target to a copper target, all features of the x-ray spectrum would change except the cutoff wavelength.
Bremsstrahlung. The bremsstrahlung is electromagnetic radiation produced by the acceleration or deceleration of an electron when deflected by strong electromagnetic fields of target high-Z (proton number) nuclei. The name bremsstrahlung comes from German, and the literal translation is ‘braking radiation.’ From classical theory, when a charged particle is accelerated or decelerated, it must radiate energy. The bremsstrahlung is one of the possible interactions of light-charged particles with matter (especially with high atomic numbers). These X-rays have a continuous spectrum. The intensity of the X-rays increases linearly with decreasing frequency, from zero at the energy of the incident electrons to the voltage on the X-ray tube. Changing the material from which the target in the tube is made does not affect the spectrum of this continuous radiation. If we were to switch from a molybdenum target to a copper target, all features of the x-ray spectrum would change except the cutoff wavelength.- Characteristic X-ray emission. If the electron has enough energy, it can knock an orbital electron out of the inner electron shell of a metal atom. Since the process leaves a vacancy in the electron energy level from which the electron came, the outer electrons of the atom cascade down to fill the lower atomic levels, and one or more characteristic X-rays are usually emitted. As a result, sharp intensity peaks appear in the spectrum at wavelengths characteristic of the material from which the anode target is made. The frequencies of the characteristic X-rays can be predicted from the Bohr model.