 A measure of radioactivity (activity) is based on counting disintegrations per second. The SI unit of activity is the becquerel (Bq), equal to one reciprocal second. The activity depends only on the number of decays per second, not on the type of decay, the energy of the decay products, or the biological effects of the radiation. It can be used to characterize the rate of emission of ionizing radiation. Specific activity is the activity per quantity of a radionuclide. Thus specific activity is defined as the activity per quantity of atoms of a particular radionuclide. It is usually given in units of Bq/g, but another commonly used unit of activity is the curie (Ci), allowing the definition of specific activity in Ci/g.
A measure of radioactivity (activity) is based on counting disintegrations per second. The SI unit of activity is the becquerel (Bq), equal to one reciprocal second. The activity depends only on the number of decays per second, not on the type of decay, the energy of the decay products, or the biological effects of the radiation. It can be used to characterize the rate of emission of ionizing radiation. Specific activity is the activity per quantity of a radionuclide. Thus specific activity is defined as the activity per quantity of atoms of a particular radionuclide. It is usually given in units of Bq/g, but another commonly used unit of activity is the curie (Ci), allowing the definition of specific activity in Ci/g.
Units of activity (the curie and the becquerel) can also be used to characterize an overall quantity of controlled or accidental releases of radioactive atoms.
Units of Activity
- Becquerel. The becquerel is the SI unit of radioactivity defined in 1974, and it is named in honor of Henri Becquerel, a French physicist who discovered radioactivity in 1896. One becquerel (1Bq) is equal to 1 disintegration per second.
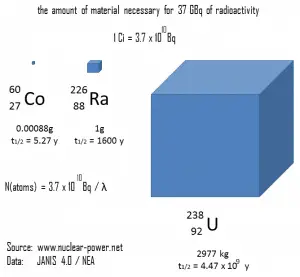
- Curie. A curie is a non-SI unit of radioactivity defined in 1910. It was originally defined as equivalent to the number of disintegrations one gram of radium-226 will undergo in one second. Currently, a curie is defined as 1Ci = 3.7 x 1010 disintegrations per second.
- Rutherford. Rutherford (symbol Rd) is also a non-SI unit defined as the activity of a quantity of radioactive material in which one million nuclei decay per second.
Decay Law
Calculations of the decay of radioactive nuclei are relatively straightforward because there is only one fundamental law governing all decay processes. The radioactive decay law can also be derived for activity calculations or mass of radioactive material calculations:
(Number of nuclei) N = N.e-λt (Activity) A = A.e-λt (Mass) m = m.e-λt
where N (number of particles) is the total number of particles in the sample, A (total activity) is the number of decays per unit time of a radioactive sample, and m is the mass of remaining radioactive material.