
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
At this place, we must distinguish between:
- Spontaneous neutron emission. Spontaneous neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus.
- Decay of free neutron. Unlike a bounded neutron, the free neutron is subject to radioactive beta decay (with a half-life of about 611 seconds). It decays into a proton, an electron, and an antineutrino (the antimatter counterpart of the neutrino, a particle with no charge and little or no mass).
- Induced neutron emission. Among nuclear reactions are also reactions in which a neutron is ejected from the nucleus and may be referred to as neutron emission reactions. These nuclear reactions are, for example:
- Scattering Reactions
- Nuclear Fission
- Photoneutrons Emission
- Other nuclear reactions (e.g., (alpha,n) reactions)
This article describes mainly spontaneous neutron emission (prompt neutron decay). This decay mode occurs only in the most neutron-rich/proton-deficient nuclides (prompt neutron decay) and also from excited states of other nuclides, as in photoneutron emission and beta-delayed neutron emission. As can be seen, if a nucleus decays via neutron emission, the atomic number remains the same, but the daughter becomes a different isotope of the same element. Nuclei that can decay by this mode lie beyond the neutron drip line. Two examples of isotopes that emit neutrons are beryllium-13 (decaying to beryllium-12 with a mean life of 2.7×10−21 s) and helium-5 (helium-4, 7×10−22 s).
Beta-delayed Neutron Emission – Delayed Neutrons
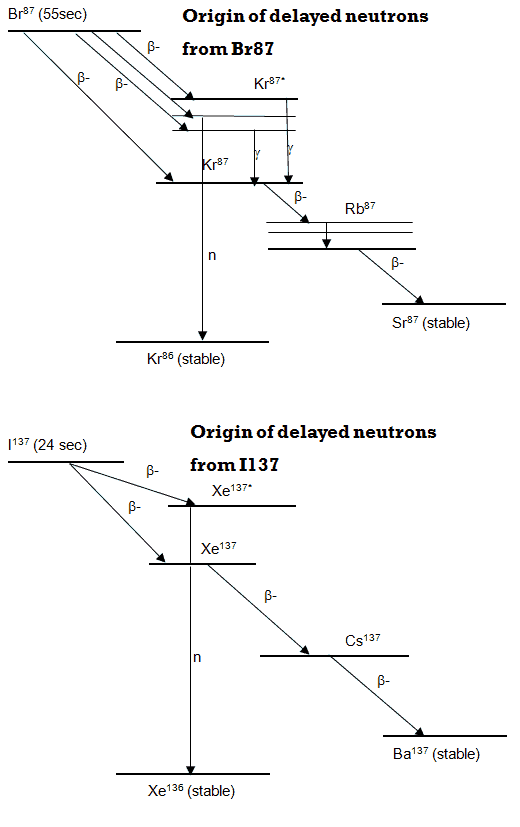
Neutron emission usually happens from nuclei in an excited state, such as the excited 87Kr* produced from the beta decay of 87Br. This isotope has a half-life of 55.6 seconds. It undergoes negative beta decay through its two main branches with emissions of 2.6 MeV and 8 MeV beta particles. This decay leads to the formation of 87Kr*, and the 87Kr* nucleus subsequently decays via two successive beta decays into the stable isotope 87Sr. But there is also one possible way for the 87Br nucleus to beta decay. The 87Br nucleus can beta decay into an excited state of the 87Kr* nucleus at an energy of 5.5 MeV, which is larger than the binding energy of a neutron in the 87Kr nucleus. In this case, the 87Kr* nucleus can undergo (with a probability of 2.5%) a neutron emission leading to the formation of a stable 86Kr isotope. The neutron emission process is controlled by the nuclear force and therefore is extremely fast, sometimes referred to as “nearly instantaneous.” The ejection of the neutron may be a product of the movement of many nucleons. Still, it is ultimately mediated by the repulsive action of the nuclear force that exists at extremely short-range distances between nucleons. The lifetime of an ejected neutron inside the nucleus before it is emitted is usually comparable to the flight time of a typical neutron before it leaves the small nuclear “potential well,” or about 10−23 seconds. As can be seen, the rate of emission of these neutrons is governed primarily by beta decay. Therefore this emission is known as beta-delayed neutron emission and is responsible for the production of delayed neutrons in nuclear reactors.
While most of the neutrons produced in fission are prompt neutrons, the delayed neutrons are important in reactor control. The presence of delayed neutrons is perhaps the most important aspect of the fission process from the viewpoint of reactor control.
See also: Precursors of Delayed Neutrons