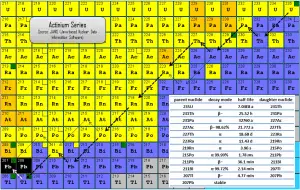
 The actinium series, also known as the actinium cascade, is one of three classical radioactive series beginning with naturally occurring uranium-235. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In the case of the actinium series, the stable nucleus is lead-207.
The actinium series, also known as the actinium cascade, is one of three classical radioactive series beginning with naturally occurring uranium-235. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In the case of the actinium series, the stable nucleus is lead-207.
Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Therefore, the mass number of the members within each series may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the actinium series is known as the 4n+3 series.
The total energy released from uranium-235 to lead-207, including the energy lost to neutrinos, is 46.4 MeV.
The activity of Natural Samples – Actinium Series
Actinium cascade influences radioactivity (disintegrations per second) of natural samples and natural materials, although, in this case, the activity of natural samples is determined primarily by uranium-238. Uranium-235, which alone constitutes 0.72% of natural uranium, has a half-life of 7.04×108 years (6.5 times shorter than the isotope 238); therefore, its abundance is lower than 238U(99.28%). All the descendants of uranium-235 and uranium-238 are present, at least transiently, in any natural uranium-containing sample, whether metal, compound or mineral. For example, pure uranium-238 is weakly radioactive (proportional to its long half-life). Still, uranium ore is about 13 times more radioactive than the pure uranium-238 metal because of its daughter isotopes (e.g., radon, radium, etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain, they also generate radon, a heavy, inert, naturally occurring radioactive gas. Moreover, the decay heat of uranium and its decay products (e.g., radon, radium, etc.) contributes to the heating of Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid.
Types of Decay in Actinium Series
Within each radioactive series, there are two main modes of radioactive decay:
- Alpha decay. Alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it heavy ionizes material and has a very short range.
- Beta-decay. Beta-decay or β decay represents the disintegration of a parent nucleus to a daughter through the emission of the beta particle. Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles have a greater range of penetration than alpha particles but still much less than gamma rays. The beta particles emitted are a form of ionizing radiation, also known as beta rays. The production of beta particles is termed beta decay.