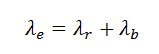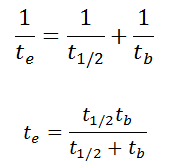In general, the effective half-life is the time taken for the amount of a specific radionuclide in the body to decrease to half its initial value from both eliminations by biological processes and radioactive decay. This variable is widely used in radiation therapy. An effective half-life of the radioactive substance will involve a decay constant that represents the sum of the biological and radioactive decay constants, as in the formula:
Besides the radioactive half-life, the effective half-life is determined by the biological half-life, which is the time taken for the amount of a particular element in the body to decrease to half of its initial value due to elimination by biological processes alone when the rate of removal is roughly exponential. The biological half-life (tbiological) can be defined for metabolites, drugs, and other substances. It is also very important in radiation protection when considering internal exposure.
If the radiation source is inside our body, we say it is internal exposure. The intake of radioactive material can occur through various pathways such as ingesting radioactive contamination in food or liquids, inhalation of radioactive gases, or through intact or wounded skin. Most radionuclides will give you much more radiation dose if they can somehow enter your body than they would if they remained outside. The biological half-life depends on the rate at which the body normally uses a particular compound of an element. Radioactive isotopes that were ingested or taken in through other pathways will gradually be removed from the body via bowels, kidneys, respiration, and perspiration. This means that a radioactive substance can be expelled before it has had the chance to decay.
As a result, the biological half-life significantly influences the effective half-life and the overall dose from internal contamination. If a radioactive compound with a radioactive half-life (t1/2) is cleared from the body with a biological half-life tb, the effective half-life (te) is given by the expression:
As can be seen, the biological mechanisms always decrease the overall dose from internal contamination. Moreover, if t1/2 is large compared to tb, the effective half-life is approximately the same as tb. For example, tritium has a biological half-life of about 10 days, while the radioactive half-life is about 12 years. On the other hand, radionuclides with very short radioactive half-lives also have very short effective half-lives. These radionuclides will deliver, for all practical purposes, the total radiation dose within the first few days or weeks after intake.
Effective Half-life of Iodine
For example, a biological half-life for iodine inside the human body is about 80 days (according to ICRP). Iodine in food is absorbed by the body and preferentially concentrated in the thyroid, where it is needed for the functioning of that gland. When 131I is present in high levels in the environment from radioactive fallout, it can be absorbed through contaminated food and accumulate in the thyroid. 131I decays with a half-life of 8.02 days with beta particle and gamma emissions. As it decays, it may cause damage to the thyroid. The primary risk from exposure to high levels of 131I is the chance of occurrence of radiogenic thyroid cancer in later life. As a result, the effective half-life of 131I is about 7.5 days, primarily by radioactive half-life. For 131I, ICRP has calculated that if you inhale 1 x 106 Bq, you will receive a thyroid dose of HT = 400 mSv (and a weighted whole-body dose of 20 mSv).
Effective Half-life of Strontium
Sr-90, Ra-226, and Pu-239 are radionuclides known as bone-seeking radionuclides. These radionuclides have long biological half-lives and are serious internal hazards. Once deposited in bone, they remain there, essentially unchanged in amount during the individual’s lifetime. The continued action of the emitted alpha particles can cause significant injury: over many years, they deposit all their energy in a tiny volume of tissue because the range of the alpha particles is very short.
Effective Half-life of Tritium
Tritium is a byproduct of nuclear reactors. The most important source (due to releases of tritiated water) of tritium in nuclear power plants stems from the boric acid, commonly used as a chemical shim to compensate for an excess of initial reactivity.
Note that tritium emits low-energy beta particles with a short-range in body tissues and, therefore, poses a risk to health as a result of internal exposure only following ingestion in drinking water or food, or inhalation or absorption through the skin. The tritium taken into the body is uniformly distributed among all soft tissues. According to the ICRP, an effective half-time of tritium is 10 days for HTO and 40 days for OBT (organically bound tritium) formed from HTO in the body of adults. Both half-lives are given primarily by biological half-life. As a result, for an intake of 1 x 109 Bq of tritium (HTO), an individual will get a whole-body dose of 20 mSv (equal to the intake of 1 x 106 Bq of 131I).
It was also shown that the biological half-time of HTO depends strongly on many variables and varies from about 4 to 18 days. During the warmer months, the average half-life is lower due to increased water intake. As well as, drinking larger amounts of alcohol will reduce the biological half-life of water in the body.