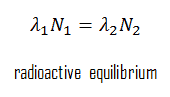In the physics of nuclear decays, a radioactive equilibrium exists when a radioactive nuclide is decaying at the same rate it is being produced. The disintegrating nucleus is usually referred to as the parent nucleus, and the nucleus remains as the daughter nucleus after the event. The daughter nucleus can either be stable or radioactive; if it is radioactive, it decays into a daughter nucleus. Thus, each radioactive parent nucleus can initiate a series of decays, with each decay product having its characteristic decay constant.
The concentration of daughter nuclei in the radioactive equilibrium depends primarily on the proportions of half-lives (or decay constants) of parent and daughter nuclei. Since the production and decay rates are equal, the number of atoms present remains constant over time. In any case, a radioactive equilibrium is not established immediately, but it only takes place after a transition period. This period is the order of a few half-lives of the longest-lived nucleus in the decay chain. In the case of radioactive decay chains, a radioactive equilibrium may be established between each member of the decay chain.
As was written, the proportionality of half-lives is a key parameter that determines the type of radioactive equilibrium:
- Radioactive equilibrium is not established when the half-life of the parent nucleus is shorter than the half-life of the daughter nucleus. In this case, the production rate and decay rate of a certain member of the decay chain cannot be equal.
- Secular radioactive equilibrium exists when the parent nucleus has an extremely long half-life. This type of equilibrium is particularly important in nature. Over the 4.5 billion years of the Earth’s history, especially uranium 238, uranium 235, and thorium 232, members of their decay chains have reached radioactive equilibria between the parent nucleus and the various descendants.
- Transient radioactive equilibrium exists when the half-life of the parent nucleus is longer than the half-life of the daughter nucleus. In this case, the parent nuclide and the daughter nuclide decay at the same rate.
Secular Equilibrium
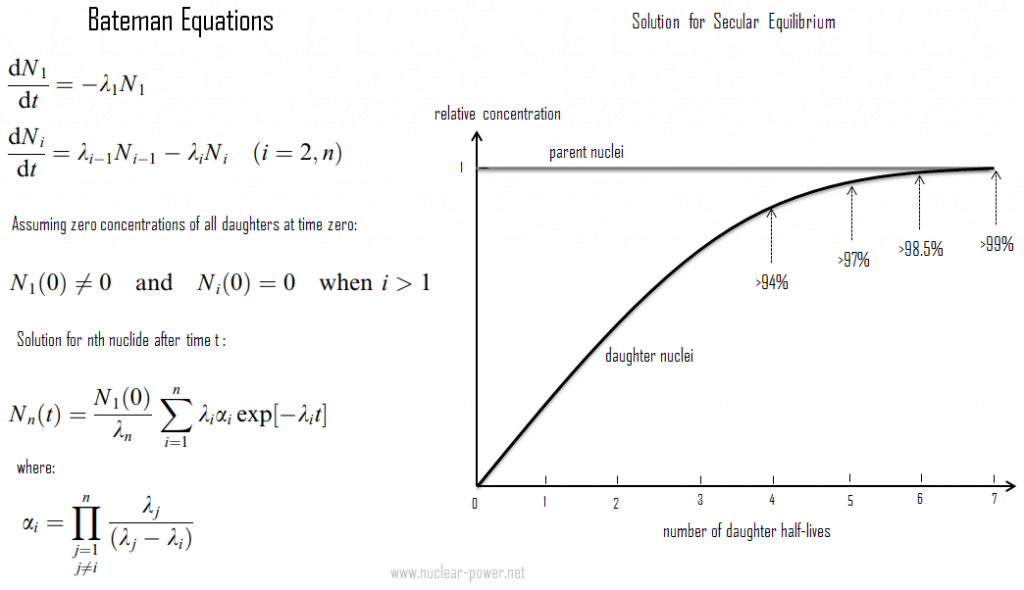
Secular radioactive equilibrium exists when the parent nucleus has an extremely long half-life. Secular equilibrium is typical for natural radioactive series, such as the thorium or uranium series. For the uranium series with uranium-238 (with a half-life of 4.47 billion years), where all of the elements in the chain are in secular equilibrium, each of the descendants has built up to an equilibrium amount, and all decay at the rate set by the original parent. The only exception is the final stable element (lead-206) on the end of the chain, and its number of atoms is constantly increasing. In any case, a radioactive equilibrium is not established immediately, but it only takes place after a transition period. This period is of a few half-lives of the longest-lived nucleus in the decay chain (234U for the uranium series; 231Pa for the actinium series). In the case of radioactive decay chains, a radioactive equilibrium may be established between each member of the decay chain.
As can be seen, the secular equilibrium is particularly important in nature. Over the 4.5 billion years of the Earth’s history, especially uranium 238, uranium 235, and thorium 232, members of their decay chains have reached radioactive equilibria between the parent nucleus and the various descendants. The half-lives of all their descendants are extremely variable, and it isn’t easy to represent a range of timescales going from individual seconds to millions of years. Since daughter radioisotopes have different half-lives, secular equilibrium is reached after some time. In the long decay chain for a naturally radioactive element, such as uranium-238, where all of the elements in the chain are in secular equilibrium, each of the descendants has built up to an equilibrium amount, and all decay at the rate set by the original parent. If and when equilibrium is achieved, each successive daughter isotope is present in direct proportion to its half-life (or decay constant).
Since its activity is inversely proportional to its half-life, each nuclide in the decay chain finally contributes as many individual transformations as the head of the chain. Radioactive cascade in the secular equilibrium significantly influences radioactivity (disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural sample, whether metal, compound or mineral. For example, pure uranium-238 is weakly radioactive (proportional to its long half-life). Still, uranium ore is about 13 times more radioactive (approximately 13 descendants in the decay chain) than the pure uranium-238 metal because of its daughter isotopes (e.g., radon, radium, etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain, they also generate radon, a heavy, inert, naturally occurring radioactive gas. Moreover, the decay heat of uranium and its decay products (e.g., radon, radium, etc.) contributes to the heating of Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid.
The secular equilibrium can occasionally be disrupted when one intermediary nucleus leaves the sample (e.g., radon released from the soil) where its ancestors are confined. These local disruptions are important to consider in the use of dating techniques. Disruption of the secular equilibrium also influences the radioactivity of fresh nuclear fuel. Most PWRs use the uranium fuel, which is in the form of uranium dioxide. But this uranium dioxide must be chemically purified, and most of its decay chain is not present in the fresh nuclear fuel.
Radioactive Equilibrium of Uranium-234
Isotope of uranium-234 is a member of the uranium series. This isotope has a half-life of only 2.46×105 years; therefore, it does not belong to primordial nuclides (unlike 235U and 238U). On the other hand, this isotope is still present in the Earth’s crust, but this is because 234U is an indirect decay product of 238U. 238U decays via alpha decay into 234U. 234U decays via alpha decay into 230Th, except a very small fraction (on the order of ppm) of nuclei decays by spontaneous fission.
In a natural sample of uranium, these nuclei are present in the unalterable proportions of the radioactive equilibrium of the 238U filiation at a ratio of one atom of 234U for about 18 500 nuclei of 238U. As a result of this equilibrium, these two isotopes (238U and 234U) contribute equally to the radioactivity of natural uranium.