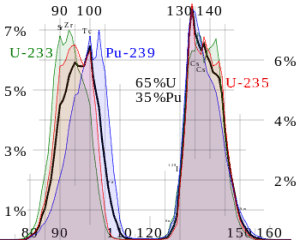
Nuclear fission fragments are the fragments left after a nucleus fissions. The average of the fragment mass is about 118, but very few fragments near that average are found. It is much more probable to break up into unequal fragments, and the most probable fragment masses are around mass 95 (Krypton) and 137 (Barium). Most of these fission fragments are highly unstable (radioactive) and undergo further radioactive decays to stabilize themselves.
Plenty of isotopes, usually neutron-rich isotopes, are produced by fission. Many of the resulting fission products have measurable thermal absorption cross-sections. Fission products are of concern in reactors primarily because they become parasitic absorbers of neutrons and result in long-term sources of heat (so-called decay heat). Their buildup in the reactor tends to reduce the multiplication factor. For that reason, these nuclei are known as neutron poisons.
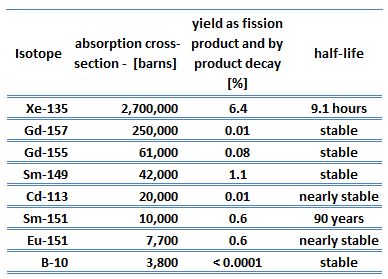
Each poison’s significance depends on the yield, decay rates, and absorption cross-section. Equilibrium is eventually reached during reactor operation if a radioisotope is produced at a constant rate. After several half-lives, the rate of decay will equal the production rate. Most fission products have a low absorption cross-section, but there are very important exceptions. These isotopes are described in the following table.
As can be seen, most of these isotopes are stable, and some undergo decay. From this point of view, we distinguish between neutron poisons, which causes reactor poisoning, and neutron poisons, which cause reactor slagging.
The isotope with a short half-life (e.g., xenon-135) is known as reactor poison, while the isotope that is long-lived or even stable is known as reactor slag. The neutron-absorbing fission products xenon-135 and samarium-149 have particular operational importance.
Their concentrations can change quickly, producing major changes in neutron absorption on a relatively short time scale. Because these two fission product poisons remove neutrons from the reactor, they will impact the thermal utilization factor and thus keff and reactivity. The importance of xenon-135 and samarium-149 is so high that they will be discussed separately.
It must be noted neutron-absorbing materials, also called neutron poisons, are intentionally inserted into some types of reactors to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant.
Reactor Slagging – Reactor Slag
As was written, an isotope long-lived or even stable is known as reactor slag. The accumulation of these parasitic absorbers is known as reactor slagging and determines the lifetime of nuclear fuel in a reactor. Their buildup causes (together with a decrease in fissile material) a continuous decrease in core reactivity.
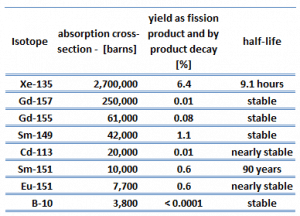 Samarium 149 belongs to this group of isotopes, but its importance is so high that it is usually discussed separately. As can be seen from the table, there are numerous other fission products that, as a result of their concentration and thermal neutron absorption cross-section, have a poisoning effect on reactor operation. Other fission products with relatively high absorption cross-sections include 83Kr, 95Mo, 143Nd, 147Pm. Individually, they are of little consequence but taken together, and they have a significant effect. These are often characterized as lumped fission product poisons.
Samarium 149 belongs to this group of isotopes, but its importance is so high that it is usually discussed separately. As can be seen from the table, there are numerous other fission products that, as a result of their concentration and thermal neutron absorption cross-section, have a poisoning effect on reactor operation. Other fission products with relatively high absorption cross-sections include 83Kr, 95Mo, 143Nd, 147Pm. Individually, they are of little consequence but taken together, and they have a significant effect. These are often characterized as lumped fission product poisons.
As was written, for fuel burnup of 40 GWd/tU, approximately 3 – 4% of the heavy nuclei are fissioned. The discharged fuel (spent nuclear fuel) still contains about 96% reusable material. It must be removed due to decreasing kinf of an assembly or in or in other words, and it must be removed due to:
- accumulation of fission products with significant absorption cross-section.
- decrease in fissile material
This is why some nuclear power plant operators use reprocessed fuel to partially replace fresh uranium fuel. Nuclear reprocessing is the technology that was developed to chemically separate and recover fissionable material from spent nuclear fuel as well as to remove these isotopes with significant absorption cross-section.