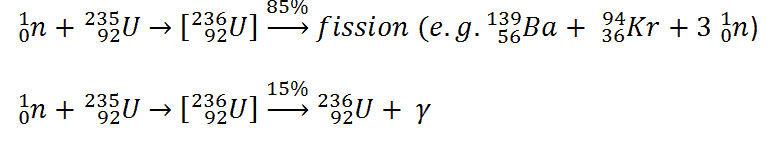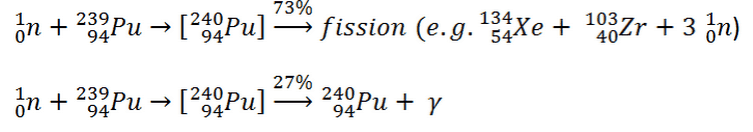 In physics, nuclear transmutation is the conversion of one chemical element or an isotope into another. In nuclear engineering, also nuclear reactors cause artificial transmutation by exposing elements to neutrons produced by fission. In nuclear reactors, the most important phenomenon is the transmutation of fertile isotopes (e.g., uranium 238) into fissile isotopes (e.g., plutonium 239). The process of the transmutation of fertile materials to fissile materials is referred to as fuel breeding.
In physics, nuclear transmutation is the conversion of one chemical element or an isotope into another. In nuclear engineering, also nuclear reactors cause artificial transmutation by exposing elements to neutrons produced by fission. In nuclear reactors, the most important phenomenon is the transmutation of fertile isotopes (e.g., uranium 238) into fissile isotopes (e.g., plutonium 239). The process of the transmutation of fertile materials to fissile materials is referred to as fuel breeding.
See also: Nuclear Breeding
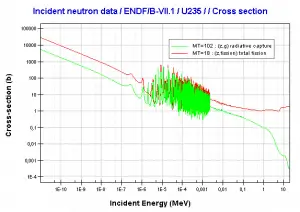
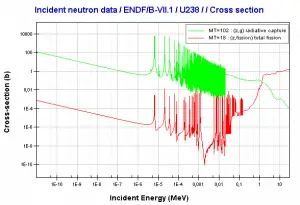
Fertile materials are not capable of undergoing fission reaction after absorbing thermal (slow or low energy) neutrons, and these materials are not capable of sustaining a nuclear fission chain reaction. There are two basic fertile materials: 238U and 232Th.
233U breeding:
232Th is the predominant isotope of natural thorium. If this fertile material is loaded in the nuclear reactor, the nuclei of 232Th absorb a neutron and become nuclei of 233Th. The half-life of 233Th is approximately 21.8 minutes. 233Th decays (negative beta decay) to 233Pa (protactinium), whose half-life is 26.97 days. 233Pa decays (negative beta decay) to 233U, which is a very good fissile material. On the other hand, proposed reactor designs must attempt to physically isolate the protactinium from further neutron capture before beta decay can occur.
239Pu breeding:
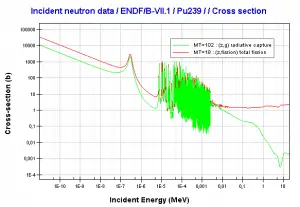
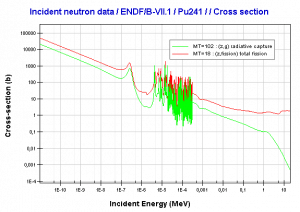
Neutron capture may also be used to create fissile 239Pu from 238U, the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.
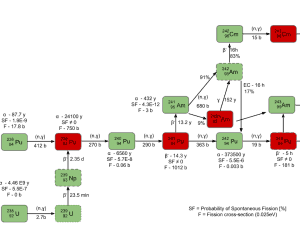
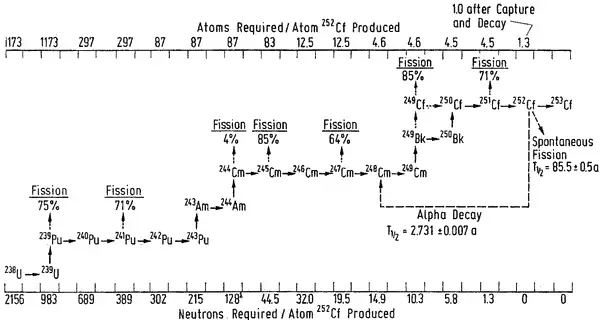
Transmutation of transuranium elements (i.e., the chemical elements with atomic numbers greater than 92 ) such as the isotopes of plutonium has the potential to help solve some problems posed by the management of radioactive waste by reducing the proportion of long-lived isotopes it contains.
Source: wikipedia.org
Fuel Depletion – Isotopic Changes
As a reactor is operated at significant power, fuel atoms are constantly consumed, resulting in the slow depletion of the fuel. It must be noted there are also research reactors, which have very low power, and the fuel in these reactors does not change its isotopic composition.
Research reactors with significant thermal power and all power reactors are subjected to significant isotopic changes. The study of these isotopic changes is known as long-term kinetics, which describes phenomena that occur over months or even years. The study of phenomena that occur for several hours to a few days, for example, effects of neutron poisons on the reactivity (i.e., Xenon poisoning or spatial oscillations), is known as medium-term kinetics.
This chapter describes the long-term kinetics of thermal reactors based on the uranium fuel cycle, in which a fuel with a large concentration of uranium-238 is used (e.g., PWRs or BWRs). Most common reactor fuels are composed of either natural or partially enriched uranium. Typically, PWRs use an enriched uranium fuel (~4% of U-235) as a fresh fuel. Exposure to neutron flux gradually depletes the uranium-235, decreasing core reactivity (compensated by control rods, chemical shim, or burnable absorbers). The initial fuel load of a new reactor core (so-called first core) is entirely fresh fuel, fuel with no plutonium or fission products present. The contribution of uranium-238 directly to fission is quite small in most thermal reactors. On the other hand, uranium-238 plays a very important role, and this chapter is primarily about this isotope.
See also: Uranium
See also: Plutonium
Evolution Equations
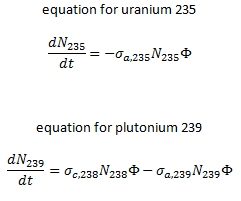
The exact evolution of isotopic changes is usually modeled mathematically by a set of differential equations known as evolution equations. These equations describe the rate of burnup of U-235, the rate of buildup of Pu-239, production of Pu-240 and Pu-241, the buildup of neutron-absorbing fission products and the overall rate of reactivity change in the reactor due to the changing composition of the fuel. The evolution equation can be constructed for each isotope. For example:
Special reference: W. M. Stacey, Nuclear Reactor Physics, John Wiley & Sons, 2001, ISBN: 0- 471-39127-1.
Special reference: Paul Reuss, Neutron Physics, EDP Sciences, 2008, ISBN: 2759800415.
Isotopic Changes – Summary

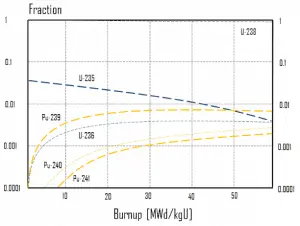
In summary, it can be seen for fuel burnup of 40 GWd/tU:
- Approximately 3 – 4% of the heavy nuclei are fissioned.
- About two-thirds of these fissions come directly from uranium 235, and the other third comes from plutonium produced from uranium 238. The contribution significantly increases as the fuel burnup increases.
- The removed fuel (spent nuclear fuel) still contains about 96% reusable material. It must be removed due to decreasing kinf of an assembly, or in other words, it must be removed due to accumulation of fission products with significant absorption cross-section.
- Discharged fuel contains about 0.8% of plutonium and about 1% of uranium 235. It must be noted there is a significant content (about 0.5%) of uranium 236, which is neither a fissile isotope nor a fertile isotope.