Atomic Number of Iodine
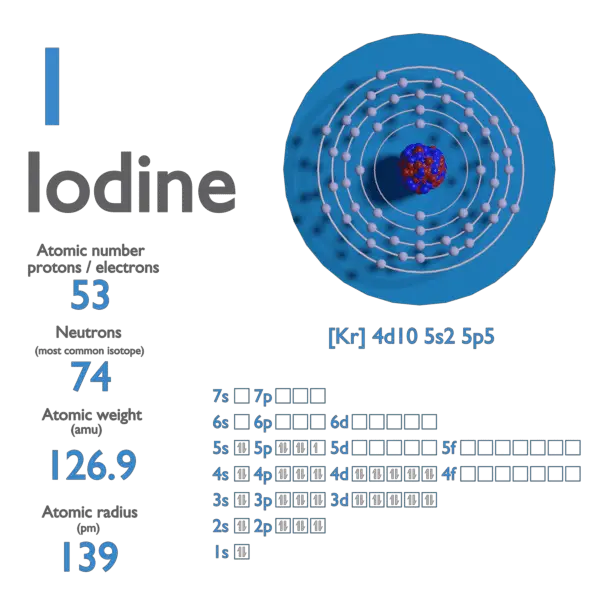
Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. The chemical symbol for Iodine is I.
Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements.
How does the atomic number determine the chemical behavior of atoms?
Atomic Mass of Iodine
Atomic mass of Iodine is 126.90447 u.
Note that each element may contain more isotopes. Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance.
The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10-24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
For 12C, the atomic mass is exactly 12u, since the atomic mass unit is defined from it. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63, and an isotopic mass in its nuclear ground state is 62.91367 u.
There are two reasons for the difference between mass number and isotopic mass, known as the mass defect:
- The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons.
- The nuclear binding energy varies between nuclei. A nucleus with greater binding energy has lower total energy, and therefore a lower mass according to Einstein’s mass-energy equivalence relation E = mc2. For 63Cu, the atomic mass is less than 63, so this must be the dominant factor.
The atomic mass number determines especially the atomic mass of atoms. The mass number is different for each different isotope of a chemical element.
How does the atomic mass determine the density of materials?
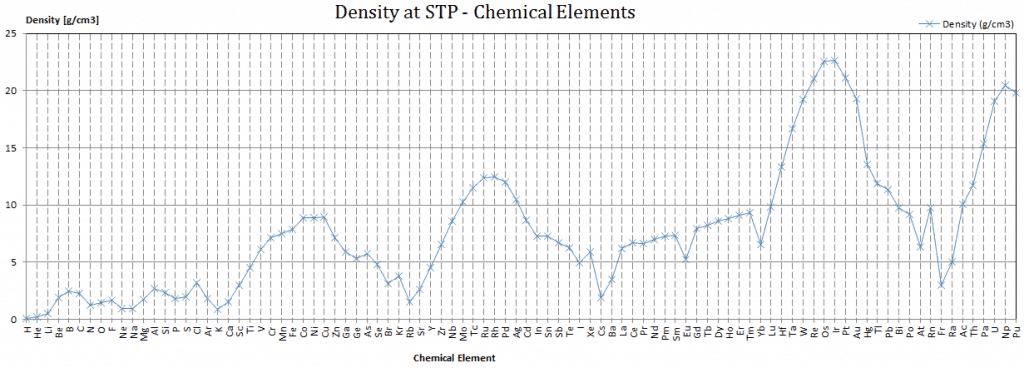
Density of Iodine
Density of Iodine is 4.94g/cm3.

Typical densities of various substances at atmospheric pressure.
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In other words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
See also: What is Density
See also: Densest Materials of the Earth
Iodine – Properties Summary
| Element | Iodine |
|---|---|
| Atomic Number | 53 |
| Symbol | I |
| Element Category | Halogen |
| Phase at STP | Solid |
| Atomic Mass [amu] | 126.90447 |
| Density at STP [g/cm3] | 4.94 |
| Electron Configuration | [Kr] 4d10 5s2 5p5 |
| Possible Oxidation States | +1,5,7/-1 |
| Electron Affinity [kJ/mol] | 295.2 |
| Electronegativity [Pauling scale] | 2.66 |
| 1st Ionization Energy [eV] | 10.4513 |
| Year of Discovery | 1811 |
| Discoverer | Courtois, Bernard |
| Thermal properties | |
| Melting Point [Celsius scale] | 113.5 |
| Boiling Point [Celsius scale] | 184 |
| Thermal Conductivity [W/m K] | 0.449 |
| Specific Heat [J/g K] | 0.214 |
| Heat of Fusion [kJ/mol] | 7.824 |
| Heat of Vaporization [kJ/mol] | 20.752 |
Iodine in Periodic Table
–
–
–