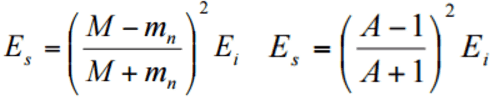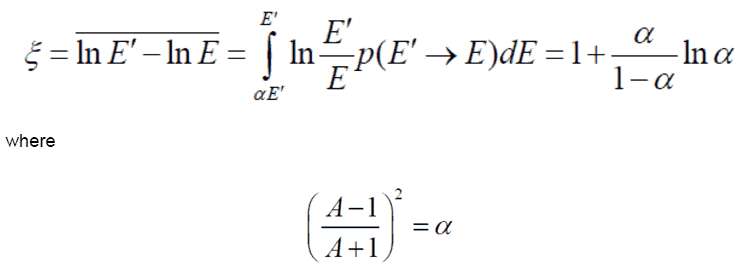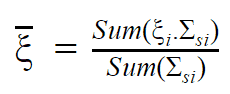Article Summary & FAQs
What is neutron moderator?
In nuclear reactors, the neutron moderator is any material used to slow down high-energy neutrons to lower energies (e.g., fission neutrons to thermal neutrons). Neutron moderators are also used for shielding of neutron radiation.
Key Facts
- Almost all prompt fission neutrons have energies between 0.1 MeV and 10 MeV.
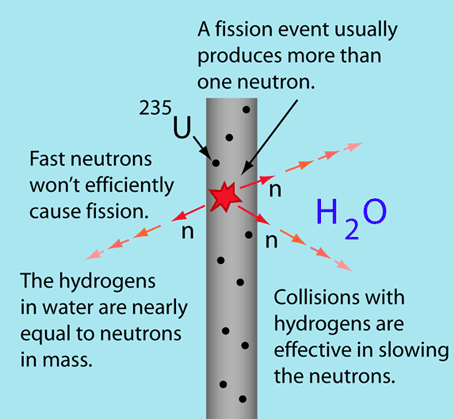
- High-energy neutrons scatter with heavy nuclei very elastically. Heavy nuclei very hard slow down a neutron let alone absorb a fast neutron.
- The probability of the fission U-235 is very small at these energies and it becomes very large at the thermal energies. This fact implies increase of multiplication factor of the reactor (i.e., lower fuel enrichment is needed to sustain chain reaction).
- For U-235, the fission cross-section for thermal neutrons is about 585 barns (for 0.0253 eV neutron). For fast neutrons its fission cross-section is on the order of barns.
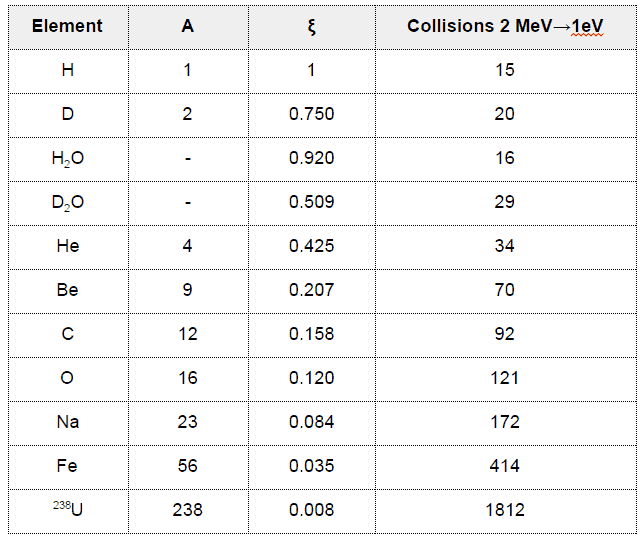
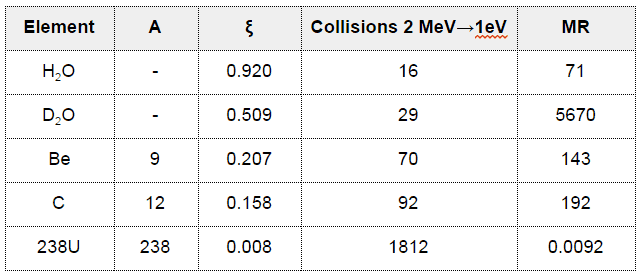
- For iron, a 2MeV neutron must undergo about 400 elastic scattering reactions to slow down to 1 eV.
- To be an effective moderator:
- The probability of elastic reaction between neutron and the nucleus must be high.
- Moderator must be made of low atomic number material.
- Moderator must have low absorption cross-section.
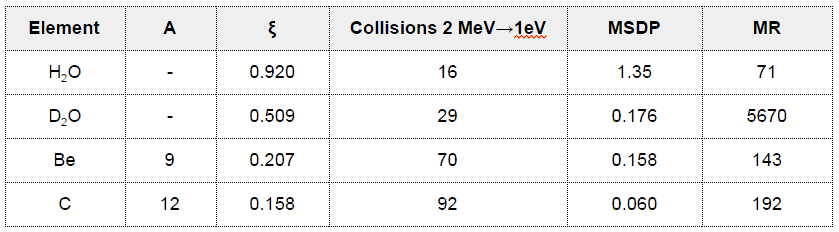
- Light water has the highest ξ and σs among the moderators (resulting in the highest MSDP) shown in the table, but its moderating ratio is low due to its relatively higher absorption cross section.
- On the other hand, heavy water has lower ξ and σs, but it has the highest moderating ratio owing to its lowest neutron absorption cross-section.
- Graphite has much heavier nuclei than hydrogen in water, despite the fact graphite has much lower ξ and σs, it is better moderator than light water due to its lower absorption cross-section compared to that of light water.
- Most common nuclear reactors are light water reactors (LWR), where light water is used as a moderator and coolant.
- In case of shielding of neutrons, the principles are the same, but higher absorption cross-section are desirable.
Neutron Moderators in Nuclear Reactors
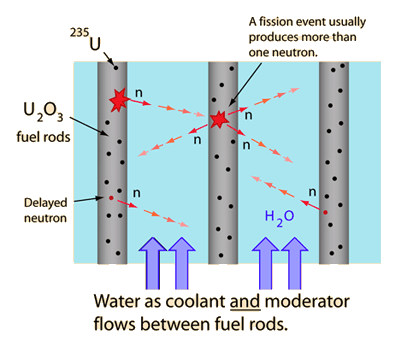
The moderator, which is of importance in thermal reactors, is used to moderate, that is, to slow down, neutrons from fission to thermal energies. The probability that fission will occur depends on incident neutron energy. Physicists calculate with fission cross-section, which determines this probability.
Nuclei with low mass numbers are most effective for this purpose, so the moderator is always a low-mass-number material. In a fast reactor there is no moderator, only fuel and coolant. The moderation of neutrons is undesirable in fast reactors. Commonly used moderators include regular (light) water (roughly 75% of the world’s reactors), solid graphite (20% of reactors) and heavy water (5% of reactors). Beryllium and beryllium oxide (BeO) have been used occasionally, but they are very costly.
Why the moderator is needed?
The probability of the fission U-235 becomes very large at the thermal energies of slow neutrons. This fact implies increase of multiplication factor of the reactor (i.e., lower fuel enrichment is needed to sustain chain reaction)
Why fast reactors don’t need moderator?
Fast reactors use fast neutrons to split uranium or plutonium nuclei. They use higher fuel enrichment to sustain chain reaction. The moderation of neutrons is undesirable in fast reactors.
Elastic Scattering and Neutron Moderators
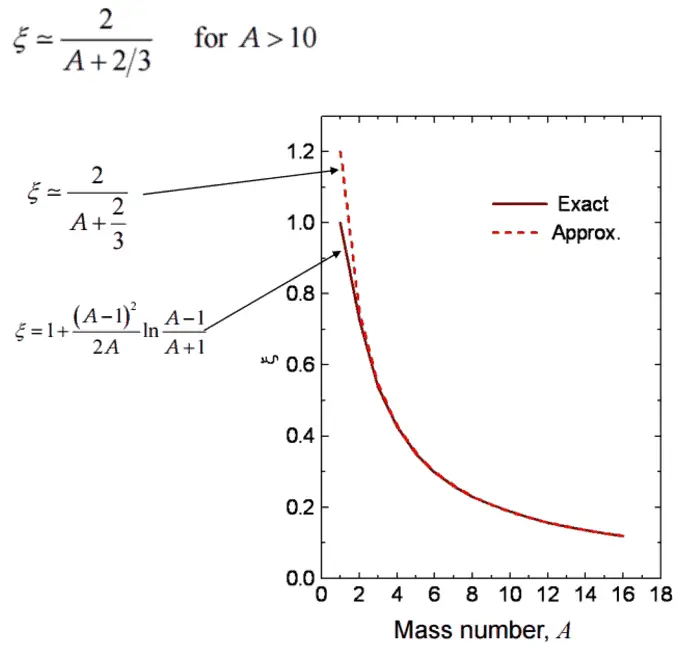
To be an effective moderator, the probability of elastic reaction between neutron and the nucleus must be high. In terms of cross-sections, the elastic scattering cross section of a moderator’s nucleus must be high. Therefore, a high elastic scattering cross-section is important, but does not describe comprehensively capabilities of moderators. In order to describe capabilities of a material to slow down neutrons, three new material variables must be defined:
- high cross-section for neutron scattering
- high energy loss per collision
- low cross-section for absorption
- high melting and boiling point
- high thermal conductivity
- high specific heat capacity
- low viscosity
- low activity
- low corrosive
- cheap