Nuclear reactors are significant sources of radiation, especially neutron radiation. At power operation, the fission reaction is responsible for the power generated in a nuclear reactor, and the fission reaction rate is proportional to the neutron flux. Fission essentially ceases when a reactor is shut down, but decay energy is still produced. The energy produced after shutdown is referred to as decay heat. Nuclear reactors are, therefore, sources of various types of radiation, with neutrons being the most important. Each type of radiation interacts in a different way. Therefore, we must describe the interaction of particles (radiation as a flow of these particles) separately. For example, charged particles with high energies can directly ionize atoms. On the other hand, electrically neutral particles interact indirectly but can also transfer some or all of their energies to matter.
This is the key feature of the categorization of radiation sources. They are usually categorized into two general types as follows:
- Charged particles (directly ionizing)
- Beta particles. Beta particles are fast electrons or positrons emitted in nuclear beta decay and energetic electrons produced by any other process. Beta radiation ionizes matter weaker than alpha radiation. On the other hand, the ranges of beta particles are longer and depend strongly on the initial kinetic energy of the particle.
- Heavy charged particles. Heavy charged particles are all energetic ions with a mass of one atomic mass unit or greater, such as protons, alpha particles (helium nuclei), or fission fragments. The stopping power of most materials is very high for alpha particles and heavy-charged particles; therefore, alpha particles have very short ranges. On the other hand, they produce massive ionization of surrounding matter.
- Neutral particles (indirectly ionizing)
- Gamma radiation (electromagnetic radiation). Gamma rays ionize matter primarily via indirect ionization. Gamma rays are very penetrating. On the other hand, their ionization is not as intense as for charged particles. Although many possible interactions are known, there are three key interaction mechanisms with the matter.
- Neutrons. Neutrons can be emitted by nuclear fission or by the decay of some radioactive atoms. Neutrons have no net electric charge. Therefore, they cannot be affected or stopped by electric forces. Neutrons ionize matter only indirectly, which makes neutrons a highly penetrating type of radiation. Neutrons scatter with heavy nuclei very elastically, and heavy nuclei very hard slow down a neutron, let alone absorb a fast neutron. An absorption of neutrons (one would say shielding) causes the initiation of certain nuclear reactions (capture, rearrangement, or even fission), which are accompanied by many other types of radiation. In short, only neutrons make matter radioactive. Therefore with neutrons, we have to shield also the other types of radiation.
Radiation-induced Crystallographic Defects
Materials in nuclear service are subjected to various types of radiation. Some of these can cause significant damage to the crystalline structure of materials. Nuclear radiation focuses large amounts of energy on highly localized areas. Damage is caused by the interaction of this energy with the nuclei and/or orbiting electrons.
As was written, charged particles with high energies can directly ionize atoms, or they can cause the excitation of surrounding electrons. The ionization and excitation dissipate much of the energy of heavier charged particles and do very little damage. This is because electrons are relatively free to move and are soon replaced. The net effect of beta and gamma radiation on metal is to generate a small amount of heat. Heavier particles, such as protons, alpha-particles, fast neutrons, and fission fragments, will usually transfer sufficient energy through elastic or inelastic collisions to remove nuclei from their lattice (crystalline) positions. This addition of vacancies and interstitial atoms causes property changes in metals.
In general, the effects of greatest interest can be described by the following groupings:
- Vacancies or Knock-ons. Vacancy defects result from a missing atom in a lattice position. The stability of the surrounding crystal structure guarantees that the neighboring atoms will not simply collapse around the vacancy. This can be caused by the direct interaction of a high-energy neutron or a fission fragment. If a target or struck nucleus gains about 25 eV of kinetic energy (25 eV to 30 eV for most metals) in a collision with a radiation particle (usually a fast neutron), the nucleus will be displaced from its equilibrium position in the crystal lattice. During lengthy irradiation (for large values of the neutron fluence), many of the displaced atoms will return to normal (stable) lattice sites (that is, partial annealing occurs spontaneously).
- Interstitials. Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being in an interstitial position instead of at its lattice position. An interstitial is formed when an atom, knocked out from its position, comes to rest at some remote point.
- Ionization. Ionization is caused by the removal of electrons from their electronic shells and has the effect of changing the chemical bonds of molecules. In metal, ionization does not cause dramatic changes in the material’s properties, and this is due to free electrons, which are typical only for a metallic bond.
- Thermal and Displacement Spikes. Thermal and displacement spikes can cause distortion that is frozen as stress in the microscopic area, and these spikes can cause a change in the material’s properties. This term identifies localized high-temperature domains caused by the deposition of energy from neutrons and fission fragments. A displacement spike occurs when many atoms in a small area are displaced by a knock-on (or cascade of knock-ons). A 1 MeV neutron may affect approximately 5000 atoms, making up one of these spikes. The presence of many displacement spikes changes the properties of the metal being irradiated, such as increased hardness and decreased ductility.
- Impurity Atoms. The capture of neutrons and nuclear reactions induced by various radiations transmutes an atom into an element foreign to the material.
- Radiation-Induced Creep. In nuclear reactors, many metal components are subjected simultaneously to radiation fields, elevated temperatures, and stress. Metal under stress at elevated temperature exhibits the phenomenon of creep, i.e., the gradual increase in strain with time. The creep of metal components at reactor operating temperatures becomes faster when exposed to a radiation field.
Neutrons with sufficient energy can disrupt materials’ atomic arrangement or crystalline structure. The influence of structural damage is most significant for metals because of their relative immunity to damage by ionizing radiation. Pressurized-water reactors operate with a higher rate of neutron impacts, and their vessels, therefore, tend to experience a greater degree of embrittlement than boiling-water reactor vessels. Many pressurized-water reactors design their cores to reduce the number of neutrons hitting the vessel wall, slowing the vessel’s embrittlement. The NRC’s regulations address embrittlement in 10 CFR Part 50, Appendix G, “Fracture Toughness Requirements,” and Appendix H, “Reactor Vessel Material Surveillance Program Requirements.” Since the reactor pressure vessel is considered irreplaceable, neutron irradiation embrittlement of pressure vessel steels is a key issue in the long-term assessment of structural integrity for life attainment and extension programs.
Radiation damage is produced when neutrons of sufficient energy displace atoms (especially in steels at operating temperatures 260 – 300°C), resulting in displacement cascades that produce large numbers of defects, vacancies, and interstitials. Although the inside surface of the RPV is exposed to neutrons of varying energies, the higher energy neutrons, above about 0.5 MeV, produce the bulk of the damage. To minimize such material degradation type and structure of the steel must be appropriately selected. Today it is known that the susceptibility of reactor pressure vessel steels is strongly affected (negatively) by the presence of copper, nickel, and phosphorus.
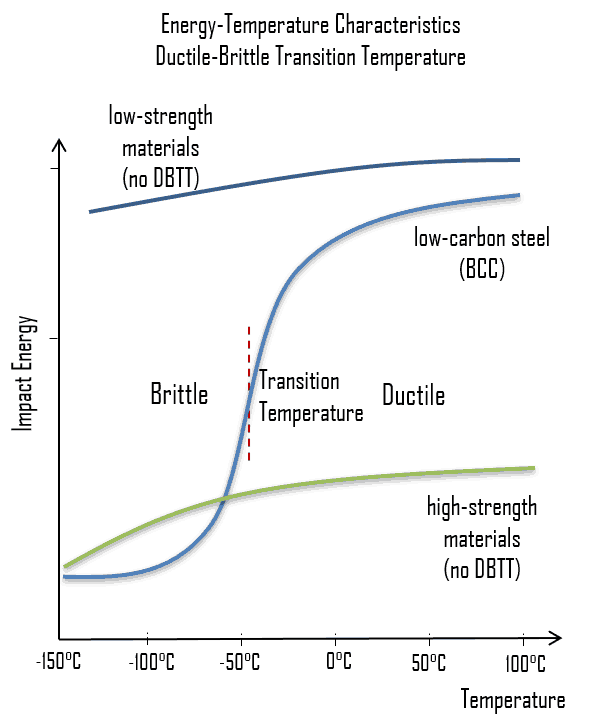 As was written, the distinction between brittleness and ductility isn’t readily apparent, especially because both ductility and brittle behavior are dependent not only on the material in question but also on the temperature (ductile-brittle transition) of the material. The effect of temperature on the nature of the fracture is of considerable importance, and many steels exhibit ductile fracture at elevated temperatures and brittle fracture at low temperatures. The temperature above which material is ductile and below which it is brittle is known as the ductile-brittle transition temperature (DBTT), nil ductility temperature (NDT), or nil ductility transition temperature. This temperature is not precise but varies according to prior mechanical and heat treatment and the nature and amounts of impurity elements. It can be determined by some form of drop-weight test (for example, the Charpy or Izod tests).
As was written, the distinction between brittleness and ductility isn’t readily apparent, especially because both ductility and brittle behavior are dependent not only on the material in question but also on the temperature (ductile-brittle transition) of the material. The effect of temperature on the nature of the fracture is of considerable importance, and many steels exhibit ductile fracture at elevated temperatures and brittle fracture at low temperatures. The temperature above which material is ductile and below which it is brittle is known as the ductile-brittle transition temperature (DBTT), nil ductility temperature (NDT), or nil ductility transition temperature. This temperature is not precise but varies according to prior mechanical and heat treatment and the nature and amounts of impurity elements. It can be determined by some form of drop-weight test (for example, the Charpy or Izod tests).
To minimize neutron fluence:
- Radial neutron reflectors are installed around the reactor core. Neutron reflectors reduce neutron leakage, reducing the neutron fluence on a reactor pressure vessel.
- Core designers design low leakage loading patterns in which fresh fuel assemblies are not situated in the peripheral positions of the reactor core.
If the metal is heated to elevated temperatures after irradiation (a form of annealing), it is found that the strength and ductility return to the same values as before irradiation. This means that radiation damage can be annealed out of metal.
See also: Ductile-brittle Transition Temperature
See also: Irradiation Embrittlement
See also: Thermal Annealing
Reactor Vessel Material Surveillance Program
Reactor vessel surveillance programs provide information on the effect of radiation on vessel materials under operating conditions. The reactor vessel surveillance program utilizes capsules located on the vessel wall directly opposite the center of the core. The capsules contain reactor vessel steel specimens obtained during vessel fabrication and are withdrawn periodically from the reactor vessel. The surveillance capsules must be located near the inside vessel wall in the beltline region so that the material specimens duplicate, to the greatest degree possible, the neutron spectrum, temperature history, and maximum neutron fluence as experienced at the reactor vessel’s inner surface. A specimen capsule containing specimens for use in Charpy V-notch, tensile, and fracture mechanics tests can be removed from the reactor during normal refueling periods.
The Charpy V-notch (CVN) technique is most commonly used. The Charpy V-notch test uses a notched sample of a defined cross-section. We use notch toughness for these dynamic loading conditions and when a notch is present. Charpy and Izod impact tests are used to measure this parameter, which is important in assessing the ductile-to-brittle transition behavior of a material. Similarly, as for tensile toughness, notch toughness is measured in units of joule per cubic meter (J·m−3) in the SI system, but in this case, we are measuring the area at the notch position.
There can also be special dosimeters, including pure nickel, copper, iron, aluminum-cobalt, or uranium-238, which can be placed in spacers specially drilled to contain the dosimeters.
According to 10 CFR 50 Appendix H, no material surveillance program is required for reactor vessels for which it can be conservatively demonstrated by analytical methods applied to experimental data and tests performed on comparable vessels, making appropriate allowances for all uncertainties in the measurements, that the peak neutron fluence at the end of the design life of the vessel will not exceed 1017 n/cm2 (E>1 MeV).
Special Reference: NUREG-1511, Reactor Pressure Vessel Status Report. U.S. Nuclear Regulatory Commission, Washington, DC, 1994.
Reactor Pressure Vessel Annealing
During the operation of a nuclear power plant, the material of the reactor pressure vessel and the material of other reactor internals are exposed to neutron radiation (especially to fast neutrons >0.5MeV), which results in localized embrittlement of the steel and welds in the area of the reactor core. This phenomenon, known as irradiation embrittlement, results in:
- Steadily increase in DBTT. It is not likely that the DBTT will approach the normal operating temperature of the steel. However, there is a possibility that when the reactor is shut down or during an abnormal cooldown, the temperature may fall below the DBTT value. At the same time, the internal pressure is still high.
- Drop in the upper shelf fracture energy. Radiation effects are also manifested by a drop in the upper shelf fracture energy and a decrease in fracture toughness.
All these effects must be monitored by plant operators. Therefore nuclear regulators require that a reactor vessel material surveillance program be conducted in water-cooled power reactors.
Once a material of RPV is degraded by radiation embrittlement (e.g., a significant increase in Charpy ductile‐brittle transition temperature or reduction of fracture toughness), thermal annealing of the RPV is the only way to recover the RPV material toughness properties.
According to 10 CFR 50.66 – Requirements for thermal annealing of the reactor pressure vessel:
“For those light water nuclear power reactors where neutron radiation has reduced the fracture toughness of the reactor vessel materials, thermal annealing may be applied to the reactor vessel to recover the fracture toughness of the material. “
Thermal annealing (“dry “method) of the reactor pressure vessel is a method by which the pressure vessel (with all reactor internals removed) is heated up to some temperature (usually between 420 – 460 °C) by use of an external heat source (electrical heaters, hot air), held for a given period (e.g., 100 – 200 hours) and then slowly cooled. The annealing equipment is usually a ring‐shaped furnace with heating elements on its external surface. The power output of installed heaters may reach up to 1 MWe. It was shown that the upper shelf recovered 100 % after 24 hours of annealing for the specially fabricated materials more rapidly than the transition temperature. Annealing for 168 hours recovered 90 % of the transition temperature shift.
Wet Annealing
The so‐called “wet” annealing method, which was applied in the USA and Belgium, is also possible. The annealing at that temperature of ~340 °C was reached without external heating but by increasing the coolant temperature achieved by the energy of the circulating pumps of the primary circuit. This type of annealing provides only partial recovery for the material due to the limitation in maximum temperature.
Special Reference: Annealing and re-embrittlement of reactor pressure vessel materials. AMES report N.19; ISSN 1018-5593. European Communities, 2008.