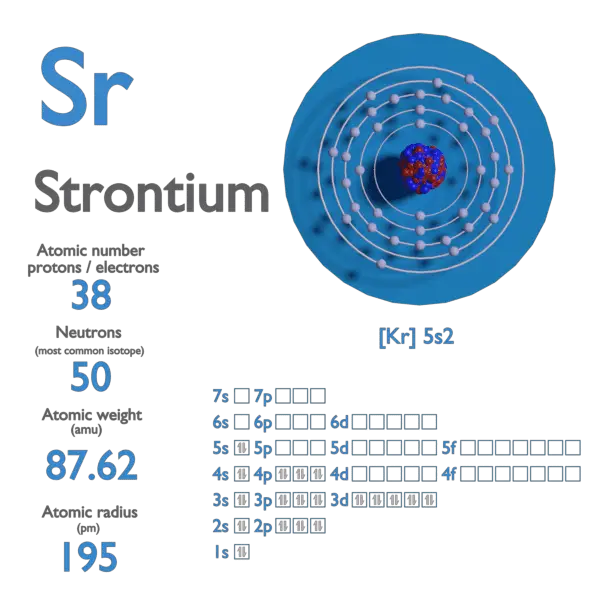
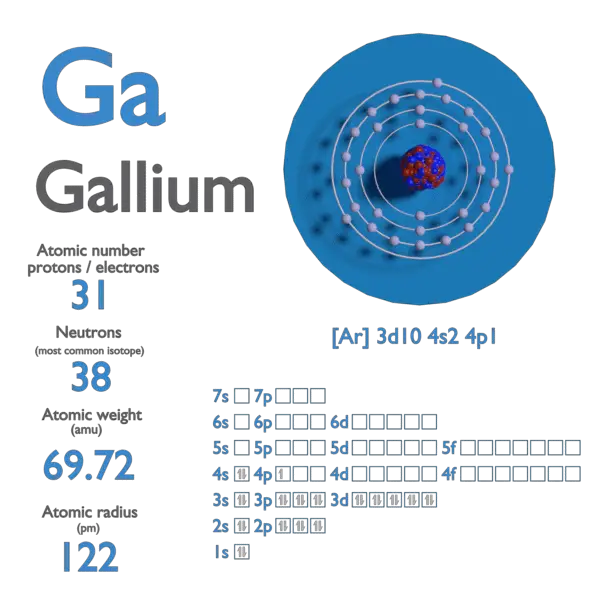
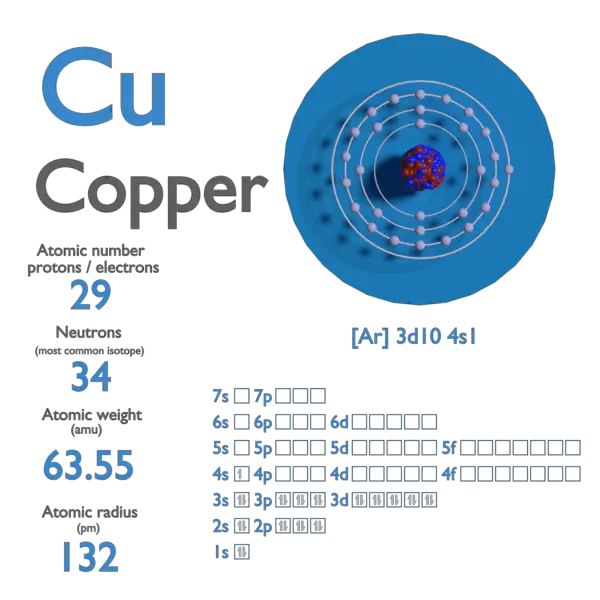
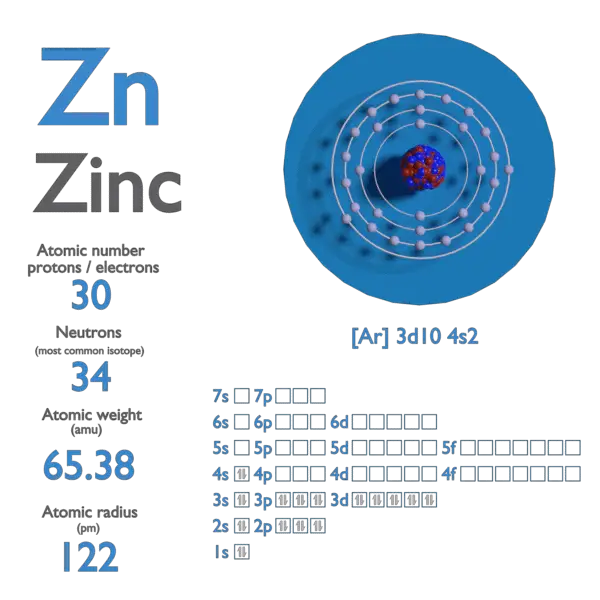
Atomic Number of Rubidium
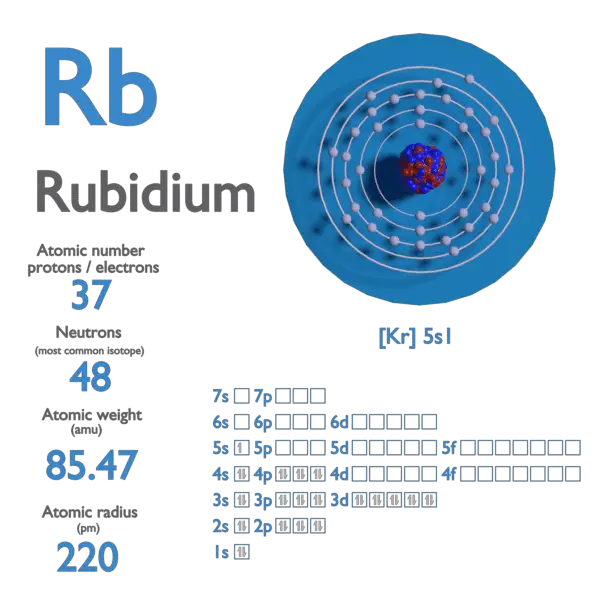
Rubidium is a chemical element with atomic number 37 which means there are 37 protons and 37 electrons in the atomic structure. The chemical symbol for Rubidium is Rb.
Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements.
How does the atomic number determine the chemical behavior of atoms?
Atomic Mass of Rubidium
Atomic mass of Rubidium is 85.4678 u.
Note that each element may contain more isotopes. Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance.
The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10-24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
For 12C, the atomic mass is exactly 12u, since the atomic mass unit is defined from it. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63, and an isotopic mass in its nuclear ground state is 62.91367 u.
There are two reasons for the difference between mass number and isotopic mass, known as the mass defect:
- The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons.
- The nuclear binding energy varies between nuclei. A nucleus with greater binding energy has lower total energy, and therefore a lower mass according to Einstein’s mass-energy equivalence relation E = mc2. For 63Cu, the atomic mass is less than 63, so this must be the dominant factor.
The atomic mass number determines especially the atomic mass of atoms. The mass number is different for each different isotope of a chemical element.
How does the atomic mass determine the density of materials?
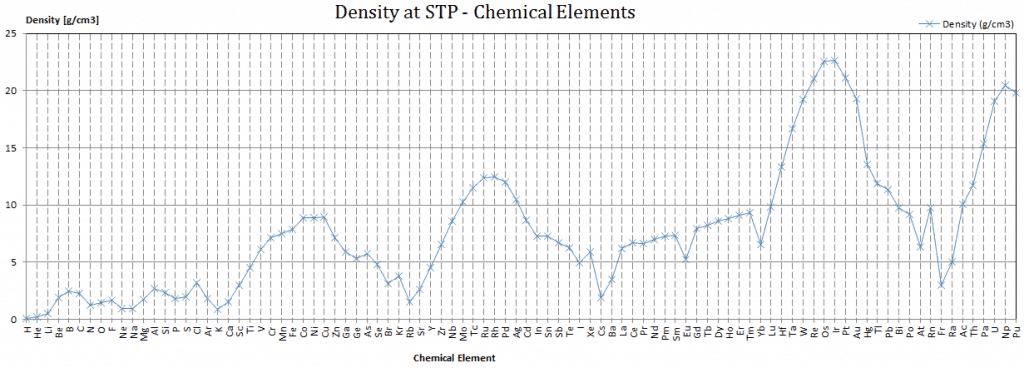
Density of Rubidium
Density of Rubidium is 1.532g/cm3.

Typical densities of various substances at atmospheric pressure.
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In other words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
See also: What is Density
See also: Densest Materials of the Earth
Rubidium – Properties Summary
| Element | Rubidium |
|---|---|
| Atomic Number | 37 |
| Symbol | Rb |
| Element Category | Alkali Metal |
| Phase at STP | Solid |
| Atomic Mass [amu] | 85.4678 |
| Density at STP [g/cm3] | 1.532 |
| Electron Configuration | [Kr] 5s1 |
| Possible Oxidation States | +1 |
| Electron Affinity [kJ/mol] | 46.9 |
| Electronegativity [Pauling scale] | 0.82 |
| 1st Ionization Energy [eV] | 4.1771 |
| Year of Discovery | 1861 |
| Discoverer | Bunsen, Robert Wilhelm & Kirchhoff, Gustav Robert |
| Thermal properties | |
| Melting Point [Celsius scale] | 39.31 |
| Boiling Point [Celsius scale] | 688 |
| Thermal Conductivity [W/m K] | 58.2 |
| Specific Heat [J/g K] | 0.363 |
| Heat of Fusion [kJ/mol] | 2.192 |
| Heat of Vaporization [kJ/mol] | 72.216 |
Rubidium in Periodic Table
–
–
–