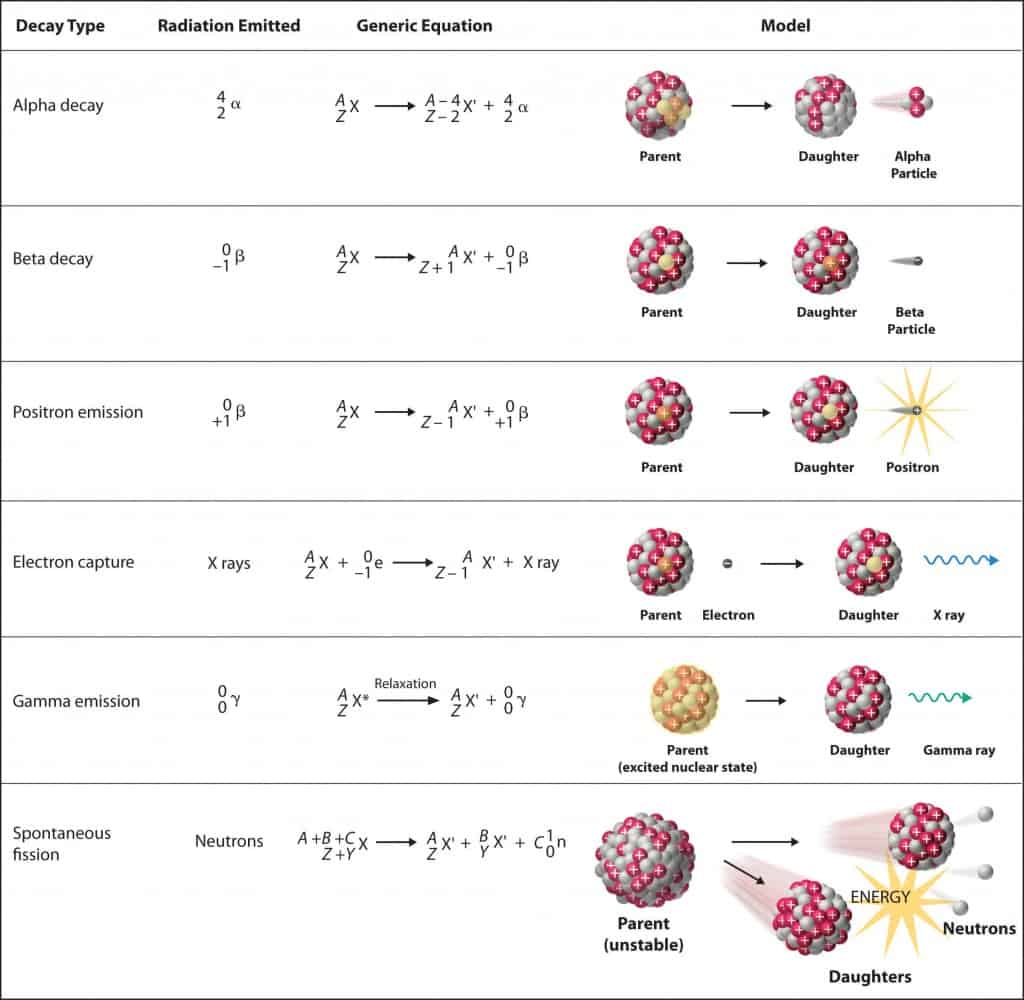
Nuclear decay (Radioactive decay) occurs when an unstable atom loses energy by emitting ionizing radiation. Radioactive decay is a random process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. There are many types of radioactive decay:
- Alpha radioactivity. Alha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it heavy ionizes material and has a very short range.
- Beta radioactivity. Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles have greater range of penetration than alpha particles, but still much less than gamma rays.The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay.
- Gamma radioactivity. Gamma rays are electromagnetic radiation of an very high frequency and are therefore high energy photons. They are produced by the decay of nuclei as they transition from a high energy state to a lower state known as gamma decay. Most of nuclear reactions are accompanied by gamma emission.
- Neutron emission. Neutron emission is a type of radioactive decay of nuclei containing excess neutrons (especially fission products), in which a neutron is simply ejected from the nucleus. This type of radiation plays key role in nuclear reactor control, because these neutrons are delayed neutrons.