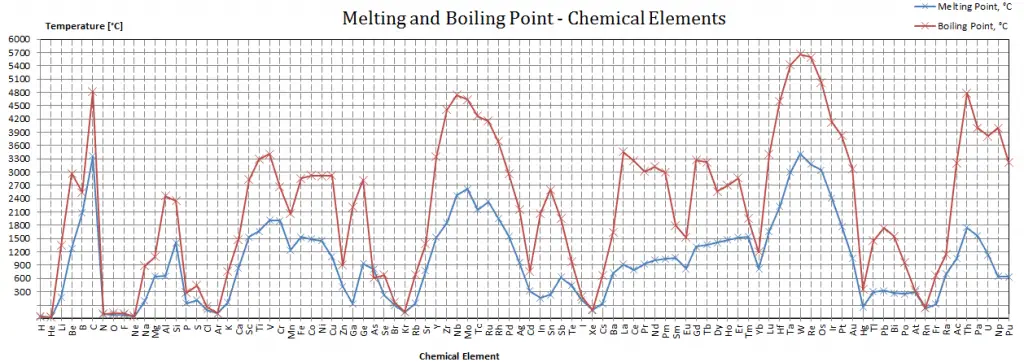
Calcium – Melting Point and Boiling Point
Melting point of Calcium is 842°C.
Boiling point of Calcium is 1484°C.
Note that these points are associated with the standard atmospheric pressure.
Boiling Point – Saturation
In thermodynamics, saturation defines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. The temperature at which vaporization (boiling) starts to occur for a given pressure is called the saturation temperature or boiling point. The pressure at which vaporization (boiling) starts to occur for a given temperature is called the saturation pressure. When considered as the temperature of the reverse change from vapor to liquid, it is called the condensation point.
Melting Point
In thermodynamics, the melting point defines a condition where the solid and liquid can exist in equilibrium. Adding heat will convert the solid into a liquid with no temperature change. The melting point of a substance depends on pressure and is usually specified at standard pressure. When considered as the temperature of the reverse change from liquid to solid, it is called the freezing point or crystallization point.
The first theory explaining the mechanism of melting in bulk was proposed by Lindemann, who used the vibration of atoms in the crystal to explain the melting transition. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as common window glass), and are typically low in energy. The motion of individual atoms, ions, or molecules in a solid is restricted to vibrational motion about a fixed point. As a solid is heated, its particles vibrate more rapidly as the solid absorbs kinetic energy. At some point, the amplitude of vibration becomes so large that the atoms start to invade the space of their nearest neighbors and disturb them, and the melting process initiates. The melting point is the temperature at which the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid.
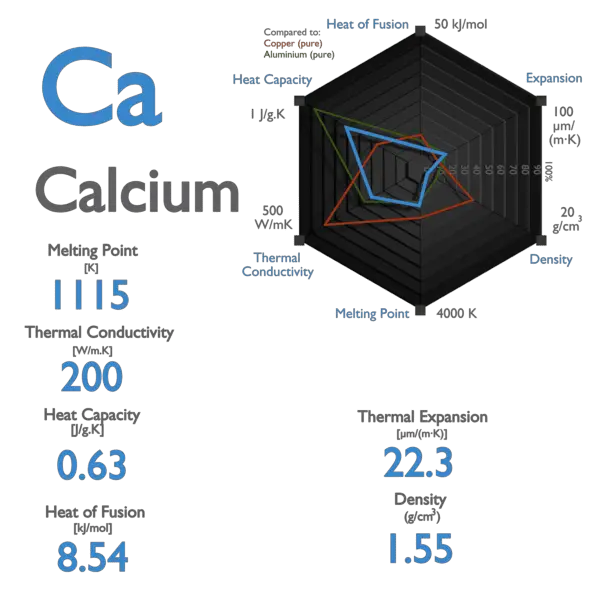
Calcium – Properties
| Element | Calcium |
|---|---|
| Atomic Number | 20 |
| Symbol | Ca |
| Element Category | Alkaline Earth Metal |
| Phase at STP | Solid |
| Atomic Mass [amu] | 40.078 |
| Density at STP [g/cm3] | 1.55 |
| Electron Configuration | [Ar] 4s2 |
| Possible Oxidation States | +2 |
| Electron Affinity [kJ/mol] | 2.37 |
| Electronegativity [Pauling scale] | 1 |
| 1st Ionization Energy [eV] | 6.1132 |
| Year of Discovery | 1808 |
| Discoverer | Davy, Sir Humphry |
| Thermal properties | |
| Melting Point [Celsius scale] | 842 |
| Boiling Point [Celsius scale] | 1484 |
| Thermal Conductivity [W/m K] | 200 |
| Specific Heat [J/g K] | 0.63 |
| Heat of Fusion [kJ/mol] | 8.54 |
| Heat of Vaporization [kJ/mol] | 153.3 |
Calcium in Periodic Table
–
–
–