Nuclear Power Plants
What is a nuclear power plant?
A nuclear power plant is a thermal power plant in which a nuclear reactor generates large amounts of heat. This heat is used to generate steam (directly or via a steam generator) which drives a steam turbine connected to a generator that produces electricity.
Key Facts
- The layout of nuclear power plants comprises two major parts: The nuclear island and the conventional (turbine) island.
- Nuclear reactors in these power plants are “only” used to generate heat. This heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity.
- The most common nuclear reactors are light water reactors (LWR), where light water is used as a moderator.
- A typical reactor may contain about 100 tonnes of enriched uranium (i.e., about 113 tonnes of uranium dioxide).
- Typical reactor nominal thermal power is about 3400MW, thus corresponding to the net electric output of 1100MW. Therefore the typical thermal efficiency of its Rankine cycle is about 33%.
- Modern power plants can operate as load-following power plants and alter their output to meet varying demands. But base load operation is the most economical and technically simple mode of operation.
- In 2011 nuclear power provided 10% of the world’s electricity. In 2007, the IAEA reported 439 nuclear power reactors in operation worldwide, operating in 31 countries.
Nuclear Physics
What is nuclear physics?
Nuclear physics is the field of physics that studies the constituents of matter (protons and neutrons) and the interactions between them. Modern nuclear physics contains especially particle physics, which is taught in close association with nuclear physics.
Key Facts
- The physical world is composed of combinations of various subatomic or fundamental particles, which are the smallest building blocks of matter.
- Fundamental particles and interactions are summarized in a theoretical model called the Standard Model.
- The atoms consist of two parts, an atomic nucleus, and an electron cloud.
- The nuclear properties (atomic mass, nuclear cross-sections) of the element are determined by the number of protons (atomic number) and the number of neutrons (neutron number).
- Nuclear stability is a concept that helps to identify the stability of an isotope. It is necessary to find the ratio of neutrons to protons to identify an isotope’s stability. You can use the ratio neutron/proton (N/Z) to determine the stability of an isotope.
- Nuclear decay (radioactive decay) occurs when an unstable atom loses energy by emitting ionizing radiation.
- Each type of particle interacts in a different way. Therefore, we must describe the interaction of particles (radiation as a flow of these particles) separately.
- A nuclear reaction is considered to be the process in which two nuclear particles (two nuclei or a nucleus and a nucleon) interact to produce two or more nuclear particles or ˠ-rays (gamma rays).
Reactor Physics
What is nuclear reactor physics?
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of neutron diffusion and fission chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy. The nuclear reactor theory is based on diffusion theory. The key term of the reactor theory is the “criticality” of the reactor, and using the term “criticality” may seem counter-intuitive to describe normalcy.
Key Facts
- In general, the study of neutron nuclear reactions and nuclear reactions is of paramount importance in the physics of nuclear reactors.
- Nuclear fission is a nuclear reaction or a decay process in which the heavy nucleus splits into smaller parts (lighter nuclei).
- In nuclear physics, the nuclear cross-section of a nucleus is commonly used to characterize the probability that a nuclear reaction will occur.
- Doppler broadening of the resonance capture cross sections of the fertile material (e.g., 238U or 240Pu) caused by the thermal motion of target nuclei in the nuclear fuel is of the highest importance for reactor stability.
- If the multiplication factor for a multiplying system is equal to 1.0, the chain reaction will be self-sustaining.
- Neutron diffusion theory deals with the spatial migration of neutrons and helps to understand the relationships between reactor size, shape, and criticality. It is also used to determine the spatial flux distributions within power reactors.
- Nuclear reactor kinetics deals with transient neutron flux changes resulting from a departure from the critical state.
- Reactor dynamics is also referred to as reactor kinetics with feedback and spatial effects.
- Basic reactor operation physics includes these topics: Reactor Startup (“criticality approach“), Control of the Reactor and Power Maneuvering, and Xenon Oscillations.
Fluid Dynamics
What is fluid dynamics?
In physics, fluid dynamics is a subdiscipline of fluid mechanics that deals with fluid flow, and fluid dynamics is one of the most important of all areas of physics.
Key Facts
- Conservation of mass in fluid dynamics states that all mass flow rates into a control volume are equal to all mass flow rates out of the control volume plus the rate of change of mass within the control volume.
- Bernoulli’s equation can be considered a statement of the conservation of energy principle appropriate for flowing fluids.
- The Reynolds number is a characteristic number used to predict whether a flow condition will be laminar or turbulent. It is defined as the ratio of inertial forces to viscous forces.
- Head loss of the hydraulic system is divided into two main categories:
- Major Head Loss – due to friction in straight pipes
- Minor Head Loss – due to components such as valves, bends…
- Darcy’s equation can be used to calculate major losses. The friction factor for fluid flow can be determined using a Moody chart.
- In fluid dynamics, drag is a force acting opposite to the relative motion of any moving object. The force a flowing fluid exerts on a body in the flow direction.
- By definition, multiphase flow is the interactive flow of two or more distinct phases with common interfaces in, say, a conduit.
- Centrifugal pumps are devices used to transport fluids by converting rotational kinetic energy to the hydrodynamic energy of the fluid flow.
Thermodynamics
What is thermodynamics?
Thermodynamics is the science that deals with energy production, storage, transfer, and conversion. It studies the effects of work, heat, and energy on a system. Although it is a very broad subject that affects most fields of science, including biology and microelectronics, we will concern mostly with large-scale observations.
Key Facts
- In physics and everyday life, a temperature is an objective comparative measurement of hot or cold based on our sense of touch. This definition is not a simple matter. The kinetic theory of gases provides a microscopic explanation of temperature. It is based on the fact that during an elastic collision between a molecule with high kinetic energy and one with low kinetic energy, part of the energy will transfer to the molecule of lower kinetic energy.
- Energy is generally defined as the potential to do work or produce heat. Sometimes it is like the “currency” for performing work. You must have the energy to accomplish work. To do 1 kilojoule of work, you must expend 1 kilojoule of energy.
- The enthalpy is the sum of the internal energy E plus the product of the pressure p and volume V.
- Four laws of thermodynamics define fundamental physical quantities (temperature, energy, and entropy) and characterize thermodynamic systems at thermal equilibrium.
- The ideal gas model is used to predict the behavior of gases and is one of the most useful and commonly used substance models ever developed.
- A thermodynamic process is defined as a change from one equilibrium macrostate to another macrostate, and the initial and final states are the defining elements of the process.
- A typical thermodynamic cycle consists of a series of thermodynamic processes transferring heat and work while varying pressure, temperature, and other state variables, eventually returning a system to its initial state.
- Today, the Rankine cycle is the fundamental operating cycle of all thermal power plants where an operating fluid is continuously evaporated and condensed.
Heat Transfer
What is heat transfer?
Heat transfer is an engineering discipline that concerns the generation, use, conversion, and exchange of heat (thermal energy) between physical systems. In power engineering, it determines key parameters and materials of heat exchangers.
Key Facts
- Heat is the amount of energy flowing from one body to another spontaneously due to their temperature difference. Heat is a form of energy, but it is energy in transit.
- Heat transfer is usually classified into various mechanisms, such as:
- Heat Conduction. Heat conduction, also called diffusion, occurs within a body or between two bodies in contact. It is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems.
- Heat Convection. Heat convection depends on a mass motion from one space region to another. Heat convection occurs when the bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid.
- Thermal Radiation. Radiation is heat transfer by electromagnetic radiation, such as sunshine, with no need for the matter to be present in the space between bodies.
- Fourier’s law of thermal conduction law states that the time rate of heat transfer through a material is proportional to the negative gradient in the temperature and the area, at right angles to that gradient, through which the heat flows.
- Newton’s law of cooling states that the rate of heat loss of a body is directly proportional to the difference in the temperatures between the body and its surroundings, provided the temperature difference is small and the nature of radiating surface remains the same.
- Stefan–Boltzmann law states that radiation heat transfer rate, q [W/m2], from a body (e.g., a black body) to its surroundings is proportional to the fourth power of the absolute temperature.
- Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common pressures, therefore, large amounts of heat can be transferred during boiling and condensation essentially at a constant temperature.
- Heat exchangers are devices that transfer thermal energy from one fluid to another without mixing the two fluids.
- To minimize heat losses in industry and also in the construction of buildings, thermal insulation is widely used. The purpose of thermal insulation is to reduce the overall heat transfer coefficient by adding material with low thermal conductivity.
Materials
What is a material?
A material is defined as a substance (most often a solid, but other condensed phases can be included) that is intended to be used for certain applications. There are many materials around us – they can be found in anything from buildings to spacecraft.
Key Facts
- Based on chemistry and atomic structure, materials are classified into three general categories:
- Metals (metallic elements),
- Ceramics (compounds between metallic and nonmetallic elements),
- Polymers (compounds composed of carbon, hydrogen, and other nonmetallic elements).
- Real materials are never perfect. Classifying crystallographic defects (microscopic defects) is frequently made according to the geometry or dimensionality of the defect.
- Key mechanical design properties are:
- Stiffness. Stiffness is the ability of an object to resist deformation in response to an applied force.
- Strength. Strength is the ability of a material to resist deformation.
- Hardness. Hardness is the ability to withstand surface indentation and scratching.
- Ductility. Ductility is the ability of a material to deform under tensile load (% elongation).
- Toughness. Toughness is the ability of a material to absorb energy (or withstand shock) and plastically deform without fracturing.
- Metal is a material (usually solid) comprising one or more metallic elements (e.g., iron, aluminium, copper, chromium, titanium, gold, nickel).
- Steels are iron-carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of nonmetallic carbon to iron trades its great ductility for greater strength.
- An alloy is a mixture of two or more materials, at least one of which is a metal. Alloys can have a microstructure consisting of solid solutions, where secondary atoms are introduced as substitutionals or interstitials in a crystal lattice.
- Non-destructive testing, NDT, is a very broad group of structural or material inspections, and as the name implies, these inspections do not destroy the material/structure being examined.
Radiation
What is ionizing radiation?
A material is defined as a substance (most often a solid, but other condensed phases can be included) that is intended to be used for certain applications. There are many materials around us – they can be found in anything from buildings to spacecraft.
Key Facts
- Ionizing radiation has different ionization mechanisms and may be grouped as:
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly by fundamental interaction through the Coulomb force if it carries sufficient kinetic energy.
- Alpha radiation. Alpha radiation consists of alpha particles at high energy/speed. The production of alpha particles is termed alpha decay.
- Beta radiation. Beta radiation consists of free electrons or positrons at relativistic speeds. The production of beta particles is termed beta decay.
- Indirectly ionizing. Indirect ionizing radiation is electrically neutral particles and therefore does not interact strongly with matter.
- Photon radiation (Gamma rays or X-rays). Photon radiation consists of high-energy photons. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus. The production of gamma rays is termed gamma decay.
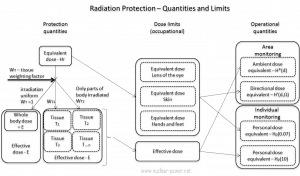
- Neutron radiation. Neutron radiation consists of free neutrons at any energy/speed. This type of radiation can be produced by nuclear reactors or in flight, and neutrons contribute 40 – 80% of the equivalent dose.
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly by fundamental interaction through the Coulomb force if it carries sufficient kinetic energy.
- There are three main types of radiation detectors, which record different types of signals.
- Counter. The activity or intensity of radiation is measured in counts per second (cps).
- Radiation Spectrometer. Spectrometers are devices designed to measure the spectral power distribution of a source.
- Dosimeter. A radiation dosimeter is a device that measures exposure to ionizing radiation.
- In general, there are two broad categories of radiation sources:
- Natural Background Radiation. Natural background radiation includes radiation produced by the Sun, lightning, primordial radioisotopes or supernova explosions, etc.
- Man-Made Sources of Radiation. Man-made sources include medical uses of radiation, residues from nuclear tests, industrial uses of radiation, etc.